The induction of preterm labor in rhesus macaques is determined by the strength of immune response to intrauterine infection
- PMID: 34495952
- PMCID: PMC8452070
- DOI: 10.1371/journal.pbio.3001385
The induction of preterm labor in rhesus macaques is determined by the strength of immune response to intrauterine infection
Abstract
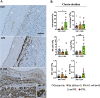
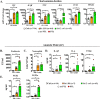
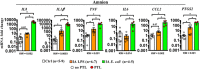
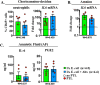
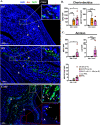
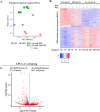
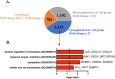
Intrauterine infection/inflammation (IUI) is a major contributor to preterm labor (PTL). However, IUI does not invariably cause PTL. We hypothesized that quantitative and qualitative differences in immune response exist in subjects with or without PTL. To define the triggers for PTL, we developed rhesus macaque models of IUI driven by lipopolysaccharide (LPS) or live Escherichia coli. PTL did not occur in LPS challenged rhesus macaques, while E. coli-infected animals frequently delivered preterm. Although LPS and live E. coli both caused immune cell infiltration, E. coli-infected animals showed higher levels of inflammatory mediators, particularly interleukin 6 (IL-6) and prostaglandins, in the chorioamnion-decidua and amniotic fluid (AF). Neutrophil infiltration in the chorio-decidua was a common feature to both LPS and E. coli. However, neutrophilic infiltration and IL6 and PTGS2 expression in the amnion was specifically induced by live E. coli. RNA sequencing (RNA-seq) analysis of fetal membranes revealed that specific pathways involved in augmentation of inflammation including type I interferon (IFN) response, chemotaxis, sumoylation, and iron homeostasis were up-regulated in the E. coli group compared to the LPS group. Our data suggest that the intensity of the host immune response to IUI may determine susceptibility to PTL.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125 e1–e15. - PubMed
-
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Drake P. Births: Final Data for 2016. Natl Vital Stat Rep. 2018;67(1):1–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

