Dynamics of G6PD activity in patients receiving weekly primaquine for therapy of Plasmodium vivax malaria
- PMID: 34495956
- PMCID: PMC8452019
- DOI: 10.1371/journal.pntd.0009690
Dynamics of G6PD activity in patients receiving weekly primaquine for therapy of Plasmodium vivax malaria
Abstract
Background: Acute Plasmodium vivax malaria is associated with haemolysis, bone marrow suppression, reticulocytopenia, and post-treatment reticulocytosis leading to haemoglobin recovery. Little is known how malaria affects glucose-6-phosphate dehydrogenase (G6PD) activity and whether changes in activity when patients present may lead qualitative tests, like the fluorescent spot test (FST), to misdiagnose G6PD deficient (G6PDd) patients as G6PD normal (G6PDn). Giving primaquine or tafenoquine to such patients could result in severe haemolysis.
Methods: We investigated the G6PD genotype, G6PD enzyme activity over time and the baseline FST phenotype in Cambodians with acute P. vivax malaria treated with 3-day dihydroartemisinin piperaquine and weekly primaquine, 0·75 mg/kg x8 doses.
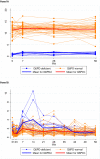
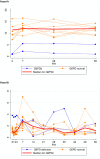
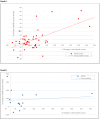
Results: Of 75 recruited patients (males 63), aged 5-63 years (median 24), 15 were G6PDd males (14 Viangchan, 1 Canton), 3 were G6PD Viangchan heterozygous females, and 57 were G6PDn; 6 patients had α/β-thalassaemia and 26 had HbE. Median (range) Day0 G6PD activities were 0·85 U/g Hb (0·10-1·36) and 11·4 U/g Hb (6·67-16·78) in G6PDd and G6PDn patients, respectively, rising significantly to 1·45 (0·36-5·54, p<0.01) and 12·0 (8·1-17·4, p = 0.04) U/g Hb on Day7, then falling to ~Day0 values by Day56. Day0 G6PD activity did not correlate (p = 0.28) with the Day0 reticulocyte counts but both correlated over time. The FST diagnosed correctly 17/18 G6PDd patients, misclassifying one heterozygous female as G6PDn.
Conclusions: In Cambodia, acute P. vivax malaria did not elevate G6PD activities in our small sample of G6PDd patients to levels that would result in a false normal qualitative test. Low G6PDd enzyme activity at disease presentation increases upon parasite clearance, parallel to reticulocytosis. More work is needed in G6PDd heterozygous females to ascertain the effect of P. vivax on their G6PD activities.
Trial registration: The trial was registered (ACTRN12613000003774) with the Australia New Zealand Clinical trials (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363399&isReview=true).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Beutler E, Duparc S, Group GPDW. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. The American journal of tropical medicine and hygiene. 2007;77(4):779–89. . - PubMed
-
- Tugwell P, Williams AO. Jaundice associated with lobar pneumonia. A clinical, laboratory and histological study. Q J Med. 1977;46(181):97–118. Epub 1977/01/01. . - PubMed
-
- Chan TK, Chesterman CN, McFadzean AJ, Todd D. The survival of glucose-6-phosphate dehydrogenase—deficient erythrocytes in patients with typhoid fever on chloramphenicol therapy. The Journal of laboratory and clinical medicine. 1971;77(2):177–84. Epub 1971/02/01. . - PubMed
-
- Alving AS, Johnson CF, Tarlov AR, Brewer GJ, Kellermeyer RW, Carson PE. Mitigation of the haemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Piasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bulletin of the World Health Organization. 1960;22:621–31. . - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

