Association between electronic nicotine delivery systems and electronic non-nicotine delivery systems with initiation of tobacco use in individuals aged < 20 years. A systematic review and meta-analysis
- PMID: 34495974
- PMCID: PMC8425526
- DOI: 10.1371/journal.pone.0256044
Association between electronic nicotine delivery systems and electronic non-nicotine delivery systems with initiation of tobacco use in individuals aged < 20 years. A systematic review and meta-analysis
Abstract
Background: This systematic review described the association between electronic nicotine delivery systems and electronic non-nicotine delivery systems (ENDS/ENNDS) use among non-smoking children and adolescents aged <20 years with subsequent tobacco use.
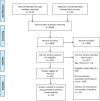
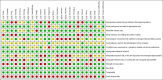
Methods: We searched five electronic databases and the grey literature up to end of September 2020. Prospective longitudinal studies that described the association between ENDS/ENNDS use, and subsequent tobacco use in those aged < 20 years who were non-smokers at baseline were included. The Joanna Briggs Institute Critical Appraisal Checklist was used to assess risk of bias. Data were extracted by two reviewers and pooled using a random-effects meta-analysis. We generated unadjusted and adjusted risk ratios (ARRs) describing associations between ENDS/ENNDS and tobacco use.
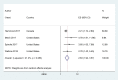
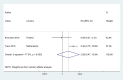
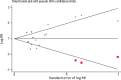
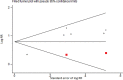
Findings: A total of 36 publications met the eligibility criteria, of which 25 were included in the systematic review (23 in the meta-analysis) after exclusion of overlapping studies. Sixteen studies had high to moderate risk of bias. Ever users of ENDS/ENNDS had over three times the risk of ever cigarette use (ARR 3·01 (95% CI: 2·37, 3·82; p<0·001, I2: 82·3%), and current cigarette use had over two times the risk (ARR 2·56 (95% CI: 1·61, 4·07; p<0·001, I2: 77·3%) at follow up. Among current ENDS/ENNDS users, there was a significant association with ever (ARR 2·63 (95% CI: 1·94, 3·57; p<0·001, I2: 21·2%)), but not current cigarette use (ARR 1·88 (95% CI: 0·34, 10·30; p = 0·47, I2: 0%)) at follow up. For other tobacco use, ARR ranged between 1·55 (95% CI 1·07, 2·23) and 8·32 (95% CI: 1·20, 57·04) for waterpipe and pipes, respectively. Additionally, two studies examined the use of ENNDS (non-nicotine devices) and found a pooled adjusted RR of 2·56 (95% CI: 0·47, 13·94, p = 0.035).
Conclusion: There is an urgent need for policies that regulate the availability, accessibility, and marketing of ENDS/ENNDS to children and adolescents. Governments should also consider adopting policies to prevent ENDS/ENNDS uptake and use in children and adolescents, up to and including a ban for this group.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- U.S. Food & Drug Administration. Vaporizers, E-Cigarettes, and other Electronic Nicotine Delivery Systems (ENDS) 2020. Available from: https://www.fda.gov/tobacco-products/products-ingredients-components/vap....
-
- Yoong SL, Tzelepis F, Wiggers J, Oldmeadow C, Chai LK, Paul C, et al. Prevalence of smoking-proxy electronic inhaling system (SEIS) use and its association with tobacco initiation in youths: a systematic review. World Health Organization, 2016.
-
- Yoong SL, Stockings E, Chai LK, Tzelepis F, Wiggers J, Oldmeadow C, et al. Prevalence of electronic nicotine delivery systems (ENDS) use among youth globally: a systematic review and meta-analysis of country level data. Australian and New Zealand Journal of Public Health. 2018;42(3):303–8. doi: 10.1111/1753-6405.12777 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

