A three-antigen Plasmodium falciparum DNA prime-Adenovirus boost malaria vaccine regimen is superior to a two-antigen regimen and protects against controlled human malaria infection in healthy malaria-naïve adults
- PMID: 34495988
- PMCID: PMC8425539
- DOI: 10.1371/journal.pone.0256980
A three-antigen Plasmodium falciparum DNA prime-Adenovirus boost malaria vaccine regimen is superior to a two-antigen regimen and protects against controlled human malaria infection in healthy malaria-naïve adults
Abstract
Background: A DNA-prime/human adenovirus serotype 5 (HuAd5) boost vaccine encoding Plasmodium falciparum (Pf) circumsporozoite protein (PfCSP) and Pf apical membrane antigen-1 (PfAMA1), elicited protection in 4/15 (27%) of subjects against controlled human malaria infection (CHMI) that was statistically associated with CD8+ T cell responses. Subjects with high level pre-existing immunity to HuAd5 were not protected, suggesting an adverse effect on vaccine efficacy (VE). We replaced HuAd5 with chimpanzee adenovirus 63 (ChAd63), and repeated the study, assessing both the two-antigen (CSP, AMA1 = CA) vaccine, and a novel three-antigen (CSP, AMA1, ME-TRAP = CAT) vaccine that included a third pre-erythrocytic stage antigen [malaria multiple epitopes (ME) fused to the Pf thrombospondin-related adhesive protein (TRAP)] to potentially enhance protection.
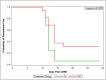
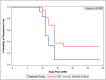
Methodology: This was an open label, randomized Phase 1 trial, assessing safety, tolerability, and VE against CHMI in healthy, malaria naïve adults. Forty subjects (20 each group) were to receive three monthly CA or CAT DNA priming immunizations, followed by corresponding ChAd63 boost four months later. Four weeks after the boost, immunized subjects and 12 infectivity controls underwent CHMI by mosquito bite using the Pf3D7 strain. VE was assessed by determining the differences in time to parasitemia as detected by thick blood smears up to 28-days post CHMI and utilizing the log rank test, and by calculating the risk ratio of each treatment group and subtracting from 1, with significance calculated by the Cochran-Mantel-Haenszel method.
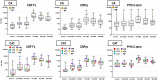
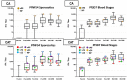
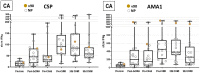
Results: In both groups, systemic adverse events (AEs) were significantly higher after the ChAd63 boost than DNA immunizations. Eleven of 12 infectivity controls developed parasitemia (mean 11.7 days). In the CA group, 15 of 16 (93.8%) immunized subjects developed parasitemia (mean 12.0 days). In the CAT group, 11 of 16 (63.8%) immunized subjects developed parasitemia (mean 13.0 days), indicating significant protection by log rank test compared to infectivity controls (p = 0.0406) and the CA group (p = 0.0229). VE (1 minus the risk ratio) in the CAT group was 25% compared to -2% in the CA group. The CA and CAT vaccines induced robust humoral (ELISA antibodies against CSP, AMA1 and TRAP, and IFA responses against sporozoites and Pf3D7 blood stages), and cellular responses (IFN-γ FluoroSpot responses to CSP, AMA1 and TRAP) that were not associated with protection.
Conclusions: This study demonstrated that the ChAd63 CAT vaccine exhibited significant protective efficacy, and confirmed protection was afforded by adding a third antigen (T) to a two-antigen (CA) formulation to achieve increased VE. Although the ChAd63-CAT vaccine was associated with increased frequencies of systemic AEs compared to the CA vaccine and, historically, compared to the HuAd5 vectored malaria vaccine encoding CSP and AMA1, they were transient and associated with increased vector dosing.
Conflict of interest statement
The authors have declared that no competing interests exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- WHO: Malaria eradication: benefits, future scenarios and feasibility. Executive summary, WHO Strategic Advisory Group on Malaria Eradication. WHO/CDS/GMP/201910 2019.
-
- Richie TL, Charoenvit Y, Wang R, Epstein JE, Hedstrom RC, Kumar S, et al..: Clinical trial in healthy malaria-naive adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA. Hum Vaccin Immunother 2012, 8:1564–1584. doi: 10.4161/hv.22129 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

