Population pharmacokinetic modeling of factor concentrates in hemophilia: an overview and evaluation of best practice
- PMID: 34496017
- PMCID: PMC8945640
- DOI: 10.1182/bloodadvances.2021005096
Population pharmacokinetic modeling of factor concentrates in hemophilia: an overview and evaluation of best practice
Abstract
The accuracy of pharmacokinetic (PK)-guided dosing depends on the clinical and laboratory data used to construct a population PK model, as well as the patient's individual PK profile. This review provides a detailed overview of data used for published population PK models for factor VIII (FVIII) and factor IX (FIX) concentrates, to support physicians in their choices of which model best suits each patient. Furthermore, to enhance detailed data collection and documentation, we do suggestions for best practice. A literature search was performed; publications describing prophylactic population PK models for FVIII and FIX concentrates based on original patient data and constructed using nonlinear mixed-effect modeling were included. The following data were collected: detailed demographics, type of product, assessed and included covariates, laboratory specifications, and validation of models. Included models were scored according to our recommendations for best practice, specifically scoring the quality of data documentation as reported. Respectively, 20 models for FVIII and 7 for FIX concentrates were retrieved. Although most models (22/27) included pediatric patients, only 4 reported detailed demographics. The wide range of body weights suggested that overweight and obese adults were represented. Twenty-six models reported the assay applied to measure factor levels, whereas only 16 models named reagents used. Eight models were internally validated using a data subset. This overview presents detailed information on clinical and laboratory data used for published population PK models. We provide recommendations on data collection and documentation to increase the reliability of PK-guided prophylactic dosing of factor concentrates in hemophilia A and B.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
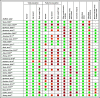
 ), incomplete (orange,
), incomplete (orange,  ), absent (red,
), absent (red,  ) or not applicable ( – ). * According to UK guidelines by Grey et al. Haemophilia 2020. † Bukkems et al. have externally validated the model by Nesterov et al.
) or not applicable ( – ). * According to UK guidelines by Grey et al. Haemophilia 2020. † Bukkems et al. have externally validated the model by Nesterov et al.References
-
- Peyvandi F, Garagiola I, Young G.. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040): 187-197. - PubMed
-
- Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculo-skeletal manifestations of haemophilia A and B. Acta Orthop Scand Suppl. 1965;(suppl. 77):3-132. - PubMed
-
- Björkman S, Blanchette VS, Fischer K, et al. ; Advate Clinical Program Group . Comparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age-related differences and implications for dose tailoring. J Thromb Haemost. 2010;8(4):730-736. - PMC - PubMed
-
- Graham A, Jaworski K.. Pharmacokinetic analysis of anti-hemophilic factor in the obese patient. Haemophilia. 2014;20(2):226-229. - PubMed
-
- Ragni MV, Croteau SE, Morfini M, Cnossen MH, Iorio A; Subcommittee on Factor VIII, Factor IX, and Rare Bleeding Disorders .Pharmacokinetics and the transition to extended half-life factor concentrates: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16(7):1437-1441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

