Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD)
- PMID: 34496032
- PMCID: PMC8425588
- DOI: 10.1002/14651858.CD013381.pub2
Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD)
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a chronic lung condition characterised by persistent respiratory symptoms and limited lung airflow, dyspnoea and recurrent exacerbations. Suboptimal therapy or non-adherence may result in limited effectiveness of pharmacological treatments and subsequently poor health outcomes.
Objectives: To determine the efficacy and safety of interventions intended to improve adherence to single or combined pharmacological treatments compared with usual care or interventions that are not intended to improve adherence in people with COPD.
Search methods: We identified randomised controlled trials (RCTs) from the Cochrane Airways Trials Register, CENTRAL, MEDLINE and Embase (search date 1 May 2020). We also searched web-based clinical trial registers.
Selection criteria: RCTs included adults with COPD diagnosed by established criteria (e.g. Global Initiative for Obstructive Lung Disease). Interventions included change to pharmacological treatment regimens, adherence aids, education, behavioural or psychological interventions (e.g. cognitive behavioural therapy), communication or follow-up by a health professional (e.g. telephone, text message or face-to-face), multi-component interventions, and interventions to improve inhaler technique.
Data collection and analysis: We used standard Cochrane methodological procedures. Working in pairs, four review authors independently selected trials for inclusion, extracted data and assessed risk of bias. We assessed confidence in the evidence for each primary outcome using GRADE. Primary outcomes were adherence, quality of life and hospital service utilisation. Adherence measures included the Adherence among Patients with Chronic Disease questionnaire (APCD). Quality of life measures included the St George's Respiratory Questionnaire (SGRQ), COPD Assessment Test (CAT) and Clinical COPD Questionnaire (CCQ).
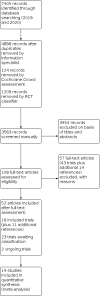
Main results: We included 14 trials (2191 participants) in the analysis with follow-up ranging from six to 52 weeks. Age ranged from 54 to 75 years, and COPD severity ranged from mild to very severe. Trials were conducted in the USA, Spain, Germany, Japan, Jordan, Northern Ireland, Iran, South Korea, China and Belgium. Risk of bias was high due to lack of blinding. Evidence certainty was downgraded due to imprecision and small participant numbers. Single component interventions Six studies (55 to 212 participants) reported single component interventions including changes to pharmacological treatment (different roflumilast doses or different inhaler types), adherence aids (Bluetooth inhaler reminder device), educational (comprehensive verbal instruction), behavioural or psychological (motivational interview). Change in dose of roflumilast may result in little to no difference in adherence (odds ratio (OR) 0.67, 95% confidence interval (CI) 0.22 to 1.99; studies = 1, participants = 55; low certainty). A Bluetooth inhaler reminder device did not improve adherence, but comprehensive verbal instruction from a health professional did improve mean adherence (prescription refills) (mean difference (MD) 1.00, 95% CI 0.46 to 1.54). Motivational interview improved mean adherence scores on the APCD scale (MD 22.22, 95% CI 8.42 to 36.02). Use of a single inhaler compared to two separate inhalers may have little to no impact on quality of life (SGRQ; MD 0.80, 95% CI -3.12 to 4.72; very low certainty). A Bluetooth inhaler monitoring device may provide a small improvement in quality of life on the CCQ (MD 0.40, 95% CI 0.07 to 0.73; very low certainty). Single inhaler use may have little to no impact on the number of people admitted to hospital compared to two separate inhalers (OR 1.47, 95% CI 0.75 to 2.90; very low certainty). Single component interventions may have little to no impact on the number of people expereincing adverse events (very low certainty evidence from studies of a change in pharmacotherapy or use of adherence aids). A change in pharmacotherapy may have little to no impact on exacerbations or deaths (very low certainty). Multi-component interventions Eight studies (30 to 734 participants) reported multi-component interventions including tailored care package that included adherence support as a key component or included inhaler technique as a component. A multi-component intervention may result in more people adhering to pharmacotherapy compared to control at 40.5 weeks (risk ratio (RR) 1.37, 95% CI 1.18 to 1.59; studies = 4, participants = 446; I2 = 0%; low certainty). There may be little to no impact on quality of life (SGRQ, Chronic Respiratory Disease Questionnaire, CAT) (studies = 3; low to very low certainty). Multi-component interventions may help to reduce the number of people admitted to hospital for any cause (OR 0.37, 95% CI 0.22 to 0.63; studies = 2, participants = 877; low certainty), or COPD-related hospitalisations (OR 0.15, 95% CI 0.07 to 0.34; studies = 2, participants = 220; moderate certainty). There may be a small benefit on people experiencing severe exacerbations. There may be little to no effect on adverse events, serious adverse events or deaths, but events were infrequently reported and were rare (low to very certainty).
Authors' conclusions: Single component interventions (e.g. education or motivational interviewing provided by a health professional) can help to improve adherence to pharmacotherapy (low to very low certainty). There were slight improvements in quality of life with a Bluetooth inhaler device, but evidence is from one study and very low certainty. Change to pharmacotherapy (e.g. single inhaler instead of two, or different doses of roflumilast) has little impact on hospitalisations or exacerbations (very low certainty). There is no difference in people experiencing adverse events (all-cause or COPD-related), or deaths (very low certainty). Multi-component interventions may improve adherence with education, motivational or behavioural components delivered by health professionals (low certainty). There is little to no impact on quality of life (low to very low certainty). They may help reduce the number of people admitted to hospital overall (specifically pharmacist-led approaches) (low certainty), and fewer people may have COPD-related hospital admissions (moderately certainty). There may be a small reduction in people experiencing severe exacerbations, but evidence is from one study (low certainty). Limited evidence found no difference in people experiencing adverse events, serious adverse events or deaths (low to very low certainty). The evidence presented should be interpreted with caution. Larger studies with more intervention types, especially single interventions, are needed. It is unclear which specific COPD subgroups would benefit, therefore discussions between health professionals and patients may help to determine whether they will help to improve health outcomes.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SJ: is employed full‐time as a systematic reviewer by a National Institute for Health Research (NIHR) Programme Grant to complete work on this review.
KP: is a senior clinical lecturer in paediatric medicine at Great Ormond Street Hospital, London, UK; has acted as a consultant on an advisory board for Respiri for development of a symptom monitoring device for asthma; and has given a lecture paid by GlaxoSmithKline. KP has also attended a Novartis severe asthma consultation to develop educational material.
RC: retired in 2018 as a general practitioner and has given lectures to primary care staff funded by GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Chiesi in the last 36 months.
AC: has COPD and is part of the patient advisory group for the current NIHR Programme Grant. He has given advice on the development of the protocol, and will provide further advice in the reviewing process.
RF: is a UK qualified general practitioner and Co‐ordinating Editor of Cochrane Airways. She is funded by grants from the NIHR.
MB: none known
Figures

























Update of
- doi: 10.1002/14651858.CD013381
Similar articles
-
Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2021 Jul 20;7(7):CD013196. doi: 10.1002/14651858.CD013196.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693988 Free PMC article.
-
Magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2022 May 26;5(5):CD013506. doi: 10.1002/14651858.CD013506.pub2. Cochrane Database Syst Rev. 2022. PMID: 35616126 Free PMC article.
-
Pulmonary rehabilitation versus usual care for adults with asthma.Cochrane Database Syst Rev. 2022 Aug 22;8(8):CD013485. doi: 10.1002/14651858.CD013485.pub2. Cochrane Database Syst Rev. 2022. PMID: 35993916 Free PMC article.
-
Individual-level interventions to reduce personal exposure to outdoor air pollution and their effects on people with long-term respiratory conditions.Cochrane Database Syst Rev. 2021 Aug 9;8(8):CD013441. doi: 10.1002/14651858.CD013441.pub2. Cochrane Database Syst Rev. 2021. PMID: 34368949 Free PMC article.
-
Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients.Cochrane Database Syst Rev. 2021 Jul 19;7(7):CD013307. doi: 10.1002/14651858.CD013307.pub2. Cochrane Database Syst Rev. 2021. PMID: 34280303 Free PMC article.
Cited by
-
Better use of inhaled medication in asthma and COPD through training, preparation and counselling: the On TRACk study protocol for a cluster randomised controlled trial.BMJ Open. 2022 Sep 6;12(9):e061266. doi: 10.1136/bmjopen-2022-061266. BMJ Open. 2022. PMID: 36691116 Free PMC article.
-
Adherence-enhancing interventions for pharmacological and oxygen therapy in patients with COPD: a systematic review and component network meta-analyses.Eur Respir Rev. 2024 Sep 4;33(173):240011. doi: 10.1183/16000617.0011-2024. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39231596 Free PMC article.
-
The evolving landscape of digital inhaler platforms and adherence support in chronic airways disease.Chron Respir Dis. 2025 Jan-Dec;22:14799731251366969. doi: 10.1177/14799731251366969. Epub 2025 Aug 8. Chron Respir Dis. 2025. PMID: 40779701 Free PMC article. Review.
-
Patients and healthcare practitioners' perspective on the development of a digital interprofessional intervention for COPD.Digit Health. 2024 Nov 3;10:20552076241288317. doi: 10.1177/20552076241288317. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 39502488 Free PMC article.
-
Enhancing potential impact of hospital discharge interventions for patients with COPD: a qualitative systematic review.BMC Health Serv Res. 2023 Jun 22;23(1):684. doi: 10.1186/s12913-023-09712-0. BMC Health Serv Res. 2023. PMID: 37349764 Free PMC article.
References
References to studies included in this review
Criner 2018 {published and unpublished data}
-
- Criner GJ, Cole T, Hahn K, Kastango K, Eudicone J, Gilbert I. A randomized clinical study to assess the impact of budesonide/formoterol (BUD/FM) pMDI medication reminders on adherence in COPD patients. European Respiratory Journal 2018;52:PA1988. - PubMed
-
- NCT02864342. Adherence study in COPD patients [A randomized clinical study to assess the impact of Symbicort® pMDI medication reminders on adherence in COPD patients]. clinicaltrials.gov/ct2/show/NCT02864342 (first received 12 August 2018).
De Tullio 1987 {published data only}
-
- De Tullio PL, Kirking DM, Arslanian C, Olson DE. Compliance measure development and assessment of theophylline therapy in ambulatory patients. Journal of Clinical Pharmacy and Therapeutics 1987;12(1):19-26. - PubMed
Grandos‐Santiago 2020 {published data only}
-
- Grandos-Santiago M, Valenza MC, Lopez-Lopez L, Prados-Roman E, Rodriguez-Torres J, Cabrera-Martos I. Shared decision-making and patient engagement program during acute exacerbation of COPD hospitalization: a randomized control trial. Patient Education and Counselling 2020;103:702-8. - PubMed
-
- Sanchez IR, Grandos-Santiago M, López-López L, Del Mar Lucena-Aguilera M, Romero-Fernández R, Valenza MC. Efficacy of a comprehensive education program including inhaler training and COPD management during hospitalization of AECOPD, a randomized controlled clinical. European Respiratory Journal 2018;52:PA3836.
Hagedorn 2013 {published data only}
-
- Hagedorn C, Kassner F, Banik N, Ntampakas P, Fielder K. Influence of salmeterol/fluticasone via single versus separate inhalers on exacerbations in severe/very severe COPD. Respiratory Medicine 2013;107(4):542-9. - PubMed
Jarab 2012 {published data only}
-
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. International Journal of Clinical Pharmacy 2012;34(1):53-62. - PubMed
Khdour 2009 {published data only}
Leiva‐Fernandez 2014 {published and unpublished data}
-
- Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D, Leiva-Fernandez F. Efficacy and safety of a multifactor intervention to improve therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): protocol for the ICEPOC study. Trials 2011;12(40):1-9. - PMC - PubMed
-
- ISRCTN18841601. Educational intervention to improve the treatment adherence in patients with chronic obstructive pulmonary disease (COPD) [Efficacy and safety of a multifactorial intervention to improve treatment adherence in patients with chronic obstructive pulmonary disease (COPD)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN18841601 (first received 28 April 2010).
-
- Leiva-Fernandez F, Barnestein-Fonseca P, Leiva- Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D. Effectiveness of a multifactorial intervention to improve adherence in patients with chronic obstructive pulmonary disease (COPD). ICEPOC study. Value in Health 2011;14:A587-8. - PMC - PubMed
-
- Leiva-Fernandez F, Leiva-Fernandez J, Prados-Torres D, Garcia-Ruiz AJ, Barnestein-Fonseca P. Factors related to adherence after a multifactorial intervention to improve it in patients with chronic obstructive pulmonary disease (COPD). ICEPOC study. Value in Health 2013;16:A373-4.
-
- Leiva-Fernandez J, Leiva-Fernandez F, Garcia-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulmonary Medicine 2014;14(70):1-12. - PMC - PubMed
Margolis 2013 {published and unpublished data}
-
- Margolis A, Young H, Lis J, Schuna A, Sorkness CA. A telepharmacy intervention to improve inhaler adherence in veterans with chronic obstructive pulmonary disease. American Journal of Health-System Pharmacy 2013;70(21):1875-6. - PubMed
-
- NCT01270594. A pilot study to evaluate a telepharmacy intervention to improve inhaler adherence in veterans with COPD. clinicaltrials.gov/show/nct01270594 (first received 5 January 2011).
Mochizuki 2013 {published data only}
-
- Mochizuki H, Nanjo Y, Takehashi H. Better adherence to a transdermal tulobuterol patch than inhaled salmeterol in elderly chronic obstructive pulmonary disease patients. Geriatrics and Gerontology International 2013;13(2):398-404. - PubMed
-
- UMIN000001503. Comparison of adherence and efficacy between inhaled salmeterol and transdermal tulobuterol patch in elderly COPD patients. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000001503 (first received 13 November 2008).
Naderloo 2018 {published data only}
-
- IRCT201604128650N7. The effect of motivational interviewing (MI) on adherence to therapy in COPD patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT201604128650N7 (first received 27 July 2016).
NCT03379233 {published data only}
-
- EUCTR2017-001593-42-BE. A 24-week randomized, single blinded study in subjects with chronic obstructive pulmonary disease (COPD) to evaluate the effect of reminders and motivational/adaptive messages on treatment adherence tracked by the Concept 2 inhaler. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2017-001593-42-BE (first received 5 March 2018).
-
- NCT03379233. A randomized study to evaluate the effect of reminder notifications and motivational/adaptive messaging on treatment adherence (ADVICE) [A 24-week randomized, controlled, multicenter, open-label study to evaluate the effect of reminder notifications and motivational/adaptive messaging on treatment adherence of COPD subjects receiving Ultibro® Breezhaler® treatment using the Concept2 inhaler for dose administration and tracking]. clinicaltrials.gov/show/nct03379233 (first received 20 December 2017).
Park 2019 {published data only}
Thom 2018 {published data only}
-
- NCT02234284. Aides in respiration health coaching for COPD (AIR). https://clinicaltrials.gov/ct2/show/results/NCT02234284 2014.
To 2020 {published data only}
-
- To KW, Lee IF, Choi KC, Cheung YT, Yu DS. An information-motivation-behavioural-based model and adherence to inhalation therapy and other health outcomes in patients with chronic obstructive pulmonary disease: a pilot randomized controlled trial. International Journal of Nursing Practice 2020;26(2):e12799. - PubMed
-
- To KW. The effects of an education-based adherence intervention on adherence of inhalation therapy among patients with chronic obstructive pulmonary disease. Unpublished doctoral thesis 2017:37-113.
Tommelein 2014 {published data only}
Wei 2014 {published data only}
References to studies excluded from this review
Alexopoulos 2016 {published data only}
Anonymous 2007 {published data only}
-
- Anonymous. Innovative inhalation system can improve compliance. Long-term therapy becomes simpler. MMW Fortschritte der Medizin 2007;149(47):55. - PubMed
Berbecaru‐Iovan 2015 {published data only}
-
- Berbecaru-Iovan S, Berbecaru-Iovan A, Stanciulescu EC, Ceausu I, Rau G, Pisoschi CG. Efficacy of indacaterol, a modern ultralong-acting bronchodilator, in elderly patients with chronic obstructive pulmonary disease. Farmacia 2015;63(2):306-12.
Bock 2012 {published data only}
-
- Bock D, Kroneberg P, Mullinger B, Roller J, Meyer M, Schierhorn K. Phase II multi-centre trial with inhaled bimosiamose in ambulatory patients with moderate to severe COPD: evaluation of dose compliance. American Journal of Respiratory and Critical Care Medicine 2012;185:A2952.
Boland 2016 {published data only}
-
- Boland M, Van Boven J, Kruis A, Chavannes N, Van Der Molen T, Goossens L, et al. COPD medication adherence and quality of life. European Respiratory Journal 2016;48:PA3961. - PubMed
Botvinikova 2003 {published data only}
-
- Botvinikova LA, Garagulya AA, Botvinikova LA, Garagulya AA. The improvement in quality of life in patients with COPD: the influence of long term regular monitoring and good compliance. European Respiratory Journal 2003;22(Suppl 45):1609.
Buist 1997 {published data only}
-
- Buist AS. The US Lung Health Study. Respirology 1997;2(4):303-7. - PubMed
Bunnag 2007 {published data only}
-
- Bunnag C, Fuangtong R, Pothirat C, Punyaratabandhu P. A comparative study of patients' preferences and sensory perceptions of three forms of inhalers among Thai asthma and COPD patients. Asian Pacific Journal of Allergy and Immunology 2007;25(2):99-109. - PubMed
Cabedo Garcia 2010 {published data only}
-
- Cabedo Garcia VR, Garces Asemany CR, Cortes Berti A, Oteo Elso JT, Ballester Salvador FJ. Effectiveness of the correct use of inhalation devices in patients with COPD: randomized clinical trial. Medicina Clinica 2010;135(13):586-91. - PubMed
Chrystyn 2014 {published data only}
Collier 2017 {published data only}
-
- Collier S, Browning D, New JP, Gibson JM, Stephens L, Diar Bakerly N, et al. Describing adherence data in a clinical effectiveness trial: the Salford lung study in COPD (SLS COPD). Thorax 2017;72:A229.
Dahl 2003 {published data only}
-
- Dahl R, Backer V, Ollgaard B, Gerken F, Kesten S. Assessment of patient performance of the HandiHaler compared with the metered dose inhaler four weeks after instruction. Respiratory Medicine 2003;97(10):1126-33. - PubMed
EUCTR2013‐001788‐21‐SK {published data only}
-
- EUCTR2013-001788-21-SK. Study to evaluate how to optimise the use of Roflumilast in subjects who have a lung disease called chronic obstructive pulmonary disease (COPD) [A multicenter, randomized, double-blind phase 3 study to evaluate tolerability and pharmacokinetics of 500µg roflumilast once daily with an up-titration regimen in COPD, including an open-label down-titration period evaluating tolerability and pharmacokinetics of 250µg roflumilast once daily in subjects not tolerating 500µg roflumilast once-daily. Evaluation of tolerability and pharmacokinetics of roflumilast (250µg and 500µg)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2013-001788-21-GB (first received 30 December 2013).
Evans 2019 {published data only}
-
- Evans CN, Volpp KG, Polsky D, Small DS, Kennedy EH, Karpink K. Prediction using a randomized evaluation of data collection integrated through connected technologies (PREDICT): design and rationale of a randomized trial of patients discharged from the hospital to home. Contemporary Clinical Trials 2019;83:53-6. - PubMed
Fischer 2000 {published data only}
-
- Fischer LR, Scott LM, Boonstra DM, DeFor TA, Cooper S, Elkema MA, et al. Pharmaceutical care for patients with chronic conditions. Journal of the American Pharmaceutical Association 2000;40(2):174-80. - PubMed
Gallefoss 1999 {published data only}
-
- Gallefoss F, Bakke P. Effect of patient education on compliance in asthmatics and chronic obstructive lung disease. American Journal of Respiratory and Critical Care Medicine 1998;157:A275. - PubMed
-
- Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? American Journal of Respiratory and Critical Care Medicine 1999;160(6):2000-5. - PubMed
Garcia‐Aymerich 2007 {published data only}
-
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101(7):1462-9. - PubMed
Hernandez 2001 {published data only}
-
- Hernandez C, Casas A, Celorrio N, Collvinent B, Farrero E, Fornas C. Impact of a home care program on adherence to treatment in patients with COPD. European Respiratory Journal 2001;18:207s.
Holzer 1988 {published data only}
-
- Holzer R. Compliance improving measures in the treatment of obstructive lung diseases. Praxis und Klinik der Pneumologie 1988;42(7):535-6. - PubMed
Kirby 2016 {published data only}
-
- Kirby S, Zhu C-Q, Kerwin EM, Stanford RH, Georges G. A randomized controlled trial comparing two dry powder inhalers: more patients with COPD prefer ellipta compared to diskus based on device-specific attributes. American Journal of Respiratory and Critical Care Medicine 2014;189:A3037.
Kripalani 2007 {published data only}
-
- Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Archives of Internal Medicine 2007;167(6):540-50. - PubMed
Mutterlein 1990 {published data only}
-
- Mutterlein R, Schmidt G, Fleischer W, Freund E. A new inhalation system for bronchodilatation. Study of the acceptance of the Ingelheim M inhaler in chronic obstructive respiratory tract diseases. Fortschritte der Medizin 1990;108(11):225-8. - PubMed
NCT00005717 {published data only}
-
- NCT00005717. Medication adherence in COPD – a self-regulation study. clinicaltrials.gov/show/nct00005717 (first received 26 May 2000).
NCT00462540 {published data only}
-
- NCT00462540. A crossover study in the treatment of patients with COPD. clinicaltrials.gov/show/NCT00462540 (first received 19 April 2007).
-
- Sutherland ER, Brazinksy S, Feldman G, McGinty J, Tomlinson L, Denis-Mize K. Nebulised formoterol effect on bronchodilation and satisfaction in COPD patients compared to QID ipratropium/albuterol MDI. Current Medical Research and Opinion 2009;25(3):653-61. - PubMed
NCT04052906 {published data only}
-
- NCT04052906. The effect on self-care and self-efficacy of inhaler training in COPD. clinicaltrials.gov/show/nct04052906 (first received 12 August 2019).
Nishimura 2011 {published data only}
-
- Nishimura N, Asakura Y, Jinta T, Suda R, Yamao S, Tomishima Y, et al. Therapeutic effect of switching tiotropium Handihaler To Respimat Soft MistTM; inhaler in the patients with COPD: the difference of adverse events and adherence to manipulations between inhaler devices. American Journal of Respiratory and Critical Care Medicine 2011;183:A1604.
Pongchaidecha 2005 {published data only}
-
- Pongchaidecha N, Trakarnkitwichit U, Pituknitinunth K. Effects of inpatient drug counseling on compliance of asthmatic and COPD patients. Thai Journal of Hospital Pharmacy 2005;15(1):28-37.
Press 2016 {published data only}
-
- NCT01456494. Teaching use of respiratory inhalers (TURI). clinicaltrials.gov/ct2/show/NCT01456494 (first received 20 October 2011).
Riar 2016 {published data only}
-
- Riar R, Carrasco L, Olibrice M, Ayinla R. Patient education with inhaler technique to prevent readmissions and emergency room (ER) visits in asthma and COPD: a quality improvement project at an inner-city hospital. Chest 2016;150(4):632A.
Schulte 2008 {published data only}
-
- Schulte M, Osseiran K, Betz R, Wencker M, Brand P, Meyer T, et al. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2008;21(4):321-8. - PubMed
Smith‐McLallen 2015 {published data only}
-
- Smith-McLallen A, Fensterheim L, Cruz S, Fu X, Solak E, Marks A, et al. A randomized clinical trial of medication therapy management in a commercial population. Journal of Managed Care and Specialty Pharmacy 2015;21(10):S77-8.
Sung 1998 {published data only}
-
- Sung SJ, Gray DR. Effectiveness and economics of a formulary switch from two separate inhalation aerosols to 2-in-1 Combivent inhaler in a veteran COPD population. American Journal of Health-System Pharmacy 1998;33:P347E.
Tack 2011 {published data only}
-
- Tack G, Tjia-Leong E, Davies L, Warburton CJ. Factors affecting inhaler choice and adherence in urban Liverpool. Thorax 2011;66:A161.
Tashkin 1991 {published data only}
-
- Nides MA, Tashkin DF, Simmons MS, Wise RA, Li VC, Rand CS. Improving inhaler adherence in a clinical trial through the use of the Nebuliser Chronolog. Chest 1993;104(2):501-7. - PubMed
-
- O'Hara P, Grill J, Rigdon MA, Connett JE, Lauger GA, Johnston JJ. Design and results of the initial intervention program for the Lung Health Study. The Lung Health Study Research Group. Preventative medicine 1993;22(3):304-15. - PubMed
-
- Rand CS, Nides M, Cowles MK, Wise RA, Connett J. Long-term metered-dose inhaler adherence in a clinical trial. The Lung Health Study Research Group. American Journal of Respiratory and Critical Care Medicine 1995;152(2):580-8. - PubMed
-
- Rand CS, Wise RA, Nides M, Simmons MS, Bleecker ER, Kusek JW, et al. Metered-dose inhaler adherence in a clinical trial. American Review of Respiratory Disease 1992;146(6):1559-64. - PubMed
-
- Simmons MS, Nides MA, Rand CS, Wise RA, Tashkin DP. Trends in compliance with bronchodilator inhaler use between follow-up visits in a clinical trial. Chest 1996;109(4):963-8. - PubMed
Trivedi 2012 {published data only}
-
- Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Annals of Behavioral Medicine 2012;44(1):66-72. - PubMed
Van Ganse 2016 {published data only}
-
- Van Ganse E, Price D. Respiratory medication adherence: toward a common language and a shared vision. Journal of Allergy and Clinical Immunology in Practice 2016;4(5):799-801. - PubMed
Van Grunsven 2000 {published data only}
-
- Van Grunsven PM, Van Schayck CP, Van Deuveren M, Van Herwaarden CLA, Akkermans RP, Van Weel C. Compliance during long-term treatment with fluticasone propionate in subjects with early signs of asthma or chronic obstructive pulmonary disease: results of the detection, intervention, and monitoring programme of COPD and asthma (DIMCA) study. Journal of Asthma 2000;37(3):225-34. - PubMed
Vanhaecht 2010 {published data only}
Van Wijk 2005 {published data only}
-
- Van Wijk BL, Klungel OH, Heerdink ER, Boer A. Effectiveness of interventions by community pharmacists to improve patient adherence to chronic medication: a systematic review. Annals of Pharmacotherapy 2005;39(2):319-28. - PubMed
Vestbo 2009 {published data only}
-
- Vestbo J, Anderson J, Calverley P, Celli B, Ferguson G, Jenkins C. Adherence to medication, mortality and severe exacerbations in the TORCH study. European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin 2008:383.
-
- Vestbo J, Anderson JA, Calverley PM, Celli B, Ferguson GT, Jenkins C, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009;64:939-43. - PubMed
-
- Vestbo J, Anderson JA, Willits LR, Celli B, Ferguson GT, Jenkins CR, et al. The TOwards a Revolution in COPD Health (TORCH) study, the effects of compliance on mortality over 3 years. American Thoracic Society International Conference; 2008 May 16-21; Toronto.
-
- Vestbo J, TORCH study group. The TORCH (towards a revolution in COPD health) survival study protocol. European Respiratory Journal 2004;24(2):206-10. - PubMed
Viejo 2001 {published data only}
-
- Viejo JI, Alcazar B, Garcia I, Guallar J, Hernandez S, Martin P, et al. A realistic study assessing inhaled treatment compliance in COPD patients. European Respiratory Journal 2001;18:25s.
Weinstein 2011 {published data only}
-
- Weinstein C, Staudinger H, Scott I, Amar NJ, LaForce C. Dose counter performance of mometasone furoate/formoterol inhalers in subjects with asthma or COPD. Respiratory Medicine 2011;105(7):979-88. - PubMed
Yildirim 2015 {published data only}
-
- Yildirim N, Demir T, Gemicioglu B, Kiyan E, Oguzulgen K, Polatli M, et al. Acute exacerbation in COPD and asthma. Tuberkuloz ve Toraks 2015;63(2):111-31. - PubMed
References to studies awaiting assessment
ACTRN12618000410257 {published data only}
-
- ACTRN12618000410257. Medication adherence management in a community pharmacy setting (AdherenciaMED): a cluster randomised controlled trial [The effect of a medication adherence management service in a community pharmacy setting on patient's adherence to medications: a cluster randomized controlled trial]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ACTRN12618000410257 (first received 21 March 2018).
Bookser 2018 {published data only}
-
- Bookser M, Drennen C, Leonard E, McConaha J. Pharmacist-led medication intervention for patients with asthma and COPD within a primary care setting. Journal of the American Pharmacists Association 2018;58(3):e24.
Davis 2016 {published data only}
Elliot 2016 {published data only}
-
- Boyd M, Waring J, Barber N, Mehta R, Chuter A, Avery AJ et al. Protocol for the new medicine service study: a randomised controlled trial and economic evaluation with qualitative appraising comparing the effectiveness and cost-effectiveness of the new medicine service in community pharmacies in England. Trials 2013;14:411. - PMC - PubMed
-
- Elliott RA, Boyd MJ, Salema NE, Davies J, Barber N, Mehta RL. Supporting adherence for people starting a new medication for a long-term condition through community pharmacies: a pragmatic randomised controlled trial of the new medicine service. British Medical Journal Quality and Safety 2016;25(10):747-58. - PubMed
-
- Elliott RA, Boyd MJ, Tanajewski L, Barber N, Gkountouras G, Avery AJ et al. New medicine service: supporting adherence in people starting a new medication for a long-term condition: 26-week follow-up of a pragmatic randomised controlled trial. British Medical Journal 2020;29(4):286-95. - PMC - PubMed
-
- Elliott RA, Tanajewski L, Gkountouras G, Avery AJ, Barber N, Mehta R et al. Cost-effectiveness of support for people starting a new medication for a long-term condition through community pharmacies: an economic evaluation of the new medicine service (NMS) compared with normal practice. Pharmacoeconomics 2017;35(12):1237-55. - PMC - PubMed
-
- NCT01635361. Understanding and appraising the new medicine service in England (NMS). https://clinicaltrials.gov/ct2/show/NCT01635361 2012.
EUCTR2016‐001435‐13‐FR {published data only}
-
- EUCTR2016-001435-13-FR. Description of the ability to learn how to handle inhaler devices in COPD [Description of the ability to learn how to handle inhaler devices in COPD – INTUITIVE]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2016-001435-13-FR (first received 17 June 2016).
Godycki‐Cwirko 2014 {published data only}
-
- Godycki-Cwirko M, Zakowska I, Kosiek K, Wensing M, Krawczyk J, Kowalczyk A. Evaluation of a tailored implementation strategy to improve the management of patients with chronic obstructive pulmonary disease in primary care: a study protocol of a cluster randomized trial. Trials 2014;15(1):109. - PMC - PubMed
Gregoriano 2015 {published data only}
-
- Gregoriano C, Dieterle T, Breitenstein AL, Durr S, Baum A, Giezendanner S, et al. Does a tailored intervention to promote adherence in patients with chronic obstructive pulmonary disease affect exacerbations? A randomised controlled trial. Respiratory Research 2019;23(6):e204. [DOI: 10.2196/resprot.7522] - DOI - PMC - PubMed
-
- Gregoriano C, Dieterle T, Flamm A-L, Durr S, Maier S, Arnet I. Objective adherence to inhaled medication and health-related outcomes in asthma and chronic obstructive pulmonary disease after an electronic monitoring intervention. Respiration 2018;95(6):486.
-
- Gregoriano C, Henny-Reinalter S, Handschin A, Flamm A-L, Dieterle T, Arnet I, et al. Patients' adherence to chronic treatment of pulmonary diseases. European Respiratory Journal 2015;46:PA1061.
-
- Gregoriano C, Henny-Reinalter S, Han d schin A, Lisa Flamm A, Dieterle T, Arnet I, et al. How do patients adhere to chronic treatment of pulmonary diseases? Baseline data from an adherence study. Praxis 2015;104:127-8.
Hesselink 2004 {published data only}
-
- Hesselink AE, Penninx BW, Van der Windt DA, Van Duin BJ, Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Education and Counselling 2004;55(1):121-8. - PubMed
ISRCTN10844309 2019 {published data only}
-
- ISRCTN10844309. Study of inhaler use in asthma and COPD [Cluster randomized controlled trial of the effectiveness and the cost-effectiveness of a pharmacist-led educational inhaler technique intervention on asthma and chronic obstructive pulmonary disease (COPD) patients (INspira)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN10844309 (first received 3 May 2019).
ISRCTN62025354 {published data only}
-
- ISRCTN62025354. Minimal interventions to improve medication adherence in people with multiple long-term conditions (MINIMA) study. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN62025354 (first received 24 January 2017).
Kristeller 2017 {published data only}
-
- Kristeller J, Snyder F, Kong F, Musheno M. Collaboration between hospital and community pharmacists to improve medication management from hospital to home. Innovations in Pharmacy 2017;8(2):1-9.
-
- NCT02047448. Improving medication adherence through a transitional care pharmacy practice model. www.clinicaltrials.gov/ct2/show/NCT02047448 (first received 28 January 2014).
Maricoto 2019 {published data only}
Navarre 2007 {published data only}
-
- Navarre M, Patel H, Johnson CE, Durance A, McMorris M, Bria W, et al. Influence of an interactive computer-based inhaler technique tutorial on patient knowledge and inhaler technique. Annals of Pharmacotherapy 2007;41:216-21. - PubMed
NCT04195191 {published data only}
-
- NCT04195191. Intervention to improve the adherence in community pharmacies [Effectiveness of an intervention to improve the adherence to treatment of new medicines from the community pharmacist]. clinicaltrials.gov/ct2/show/NCT04195191 (first received 11 December 2019).
Nimmo 1993 {published data only}
-
- Nimmo CJ, Chen DN, Martinusen SM, Ustad TL, Ostrow DN. Assessment of patient acceptance and inhalation technique of a pressurized aerosol inhaler and two breath-actuated devices. Annals of Pharmacotherapy 1993;27(7):922-7. - PubMed
NTR5187 {published data only}
-
- Kemerink A, Steenhuis LH, Klemmeier TJ, Vroegop JS, Schokker S. Inhalation technique education in asthma or COPD: the value of a visual instruction card. European Respiratory Journal 2016;48:PA5006. [DOI: 10.1183/13993003congress-2016.PA5006] - DOI
O'Dwyer 2016 {published data only}
-
- NCT02203266. Teaching inhaler use with the INCA device in a community pharmacy setting [A randomised, parallel-group, multi-centre trial using a novel INCA tracker device to measure and monitor compliance and technique of Seretide Diskus inhaler in a community pharmacy setting]. www.clinicaltrials.gov/ct2/show/NCT02203266 (first received 29 July 2014).
-
- O'Dwyer S, Greene G, MacHale E, Cushen B, Sulaiman I, Boland F, et al. Personalised biofeedback on inhaler adherence and technique by community pharmacists: a cluster randomised clinical trial. Journal of Allergy and Clinical Immunology Practice 2020;8:635-44. - PubMed
Qin 2016 {published data only}
-
- Qin Q, Chen R, Lei W, Bian Y-C, Gu B-C, Bao J-A. Evaluation and analysis of inhaler drug device adherence in asthma and COPD patients. Chinese Pharmaceutical Journal 2016;51(5):413-6.
RBR‐5bw2wt {published data only}
-
- RBR-5bw2wt. The role of the pharmacist in the team care of hospitalised patients with chronic bronchitis or emphysema [Pharmaceutical care programme for inpatients with chronic obstructive pulmonary disease in a tertiary teaching hospital in southern Brazil – PHARBE: PHARmaceutical Care Programme for Inpatients with COPD in a Tertiary Teaching Hospital in Southern Brazil]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=RBR-5bw2wt (first received 1 May 2012).
SAM30001 GSK {published data only}
-
- SAM30001 GSK. Compliance in asthma and COPD. www.gsk-studyregister.com/en/trial-details/?id=SAM30001#documents-section (first received 1 May 1999).
Serra‐Batlles 2002 {published data only}
-
- Serra-Batlles J, Plaza V, Badiola C, Morejon E, Inhalation Devices Study Group, Morejon E, et al. Patient perception and acceptability of multidose dry powder inhalers: a randomized crossover comparison of Diskus/Accuhaler with Turbuhaler. Journal of Aerosol Medicine 2002;15(1):59-64. - PubMed
Suhaj 2016 {published data only}
-
- Abdulsalim S, Unnikrishnan K, Manu MK, Alrasheedy AA, Godman B, Morisky DE. Structured pharmacist-led intervention programme to improve medication adherence in COPD patients: a randomised controlled trial. Research in Social and Administrative Pharmacy 2018;14:909-14. - PubMed
-
- Suhaj A, Manu MK, Unnikrishnan MK, Vijayanarayana K, Mallikarjuna CR. Effectiveness of clinical pharmacist intervention on health-related quality of life in chronic obstructive pulmonary disorder patients – a randomized controlled study. Journal of Clinical Pharmacy and Therapeutics 2016;41(1):78-83. - PubMed
-
- Suhaj A, Unnikrishnan M, Mohan MK, Rao CM, Vijayanarayana K. The effectiveness of clinical pharmacist intervention on health related quality of life in chronic obstructive pulmonary disorder patients – a randomized controlled study. Respirology 2015;20:58. - PubMed
References to ongoing studies
ISRCTN10567920 {published data only}
-
- ISRCTN10567920. Maximising adherence and gaining new information for your COPD [A pragmatic, cluster randomized trial evaluating the impact of an enhanced adherence package (dual bronchodilator + add-on + app) on time to treatment failure and other clinical outcomes in exacerbating COPD patients with poor adherence to mono or dual therapy over one year]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN10567920 (first received 25 July 2019).
ISRCTN77785397 {published data only}
-
- ISRCTN77785397. Using the internet to help individuals stay healthy and prevent further reductions in health from existing chronic diseases [Maximizing the effects of self-management interventions on chronic disease outcomes: the development of a chronic obstructive pulmonary disease (COPD) web-based patient portal]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN10567920 (first received 8 November 2011).
Additional references
Agh 2015
-
- Ágh T, Dömötör P, Bártfai Z, Inotai A, Fujsz E, Mészáros Á. Relationship between medication adherence and health related quality of life in subjects with COPD: a systematic review. Respiratory Care 2015;60(2):297-303. - PubMed
Blackstock 2016
-
- Blackstock FC, ZuWallack R, Nici L, Lareau SC. Why don't our patients with chronic obstructive pulmonary disease listen to us? Annals of the American Thoracic Society 2016;13(3):317-23. - PubMed
Blakey 2018
BMJ 2012
-
- Healthcare professionals as bad as patients at good respiratory inhaler technique: editorial. www.sciencedaily.com/releases/2012/10/121003195134.htm (accessed prior to 19 May 2021).
Brandstetter 2017
Bryant 2013
Chen 2017
Cochrane Airways 2019
-
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 12 March 2019).
COPD Foundation 2018
-
- COPD Foundation. What is COPD? www.copdfoundation.org/What-is-COPD/Understanding-COPD/What-is-COPD.aspx (accessed 5 February 2019).
De Geest 2018
Duncan 2015
-
- Duncan D. Medication adherence in chronic obstructive pulmonary disease. Practice Nursing 2015;26(10):493-8.
Ejiofor 2013
Furmedge 2018
-
- Furmedge DS, Stevenson JM, Schiff R, Davies JG. Evidence and tips on the use of medication compliance aids. BMJ 2018;362:k2801. - PubMed
GBD 2015 Chronic Respiratory Disease Collaborators
-
- Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease study 2015. Lancet Respiratory Medicine 2017;5:691-706. - PMC - PubMed
GBD 2015 Incidence and Prevalence Collaborators
-
- Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. - PMC - PubMed
GOLD 2021
-
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2021 global strategy for the diagnosis, management, and prevention of COPD. goldcopd.org/ (accessed 5 May 2021).
Gore 1981
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 5 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Herath 2018
Higgins 2019
-
- Higgins JP, Thomas J, Chandler K, Cumpston M, Li T, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Second edition. Chichester (UK): John Wiley & Sons, 2019. - PubMed
Humenberger 2018
Jones 2005
-
- Jones W. St. George’s Respiratory Questionnaire: MCID. Journal of Chronic Obstructive Pulmonary Disease 2005;2:75-9. - PubMed
Jordan 2015
Klijn 2017
Kocks 2006
Lorig 2001
-
- Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program for patients with chronic disease. Effective Clinical Practice 2001;4:256-62. - PubMed
Marshall 2018
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. Global Evidence Summit; 2017 Sept 13-16; Cape Town, South Africa.
Moher 2009
NHLBI 1982
-
- National Heart, Lung and Blood Institute Working Group on Patient Compliance. Management of patient compliance in the treatment of hypertension. Hypertension 1982;4:415-53. - PubMed
NICE 2018
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [CG101]. www.nice.org.uk/guidance/cg101 (accessed prior to 5 March 2019). - PubMed
Nieuwlaat 2014
Noel‐Storr 2018
-
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford, UK. 2018.
Normansell 2017
Poole 2019
Qaseem 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155:179-91. - PubMed
Quaderi 2018
Restrepo 2008
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rootmensen 2010
-
- Rootmensen GN, Van Keimpema ARJ, Jansen HM, Haan RJ. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2010;23(5):323-8. - PubMed
Sabaté 2003
-
- Sabaté E. Adherence to long-term therapies: evidence for action. https://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf?se... [accessed September 2021] 2003.
Sulaiman 2017
-
- Sulaiman I, Cushen B, Greene G, Seheult J, Seow D, Rawat F, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2017;195(10):1333-43. - PubMed
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Van Boven 2014
-
- Van Boven JF, Chavannes NH, Van der Molen T, Rutten-van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respiratory Medicine 2014;108(1):103-13. - PubMed
Vestbo 2013
-
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2013;187(4):347-65. - PubMed
Vogelmeier 2017
-
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Borbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2017;195(5):557-82. - PubMed
Vrijens 2016
-
- Vrijens B, Dima AL, Van Ganse E, Van Boven JF, Eakin MN, Foster JM, et al. What we mean when we talk about adherence in respiratory medicine. Journal of Allergy and Clinical Immunology: In Practice 2016;4(5):802-12. - PubMed
Walters 2014
Welling 2015
-
- Welling JB, Hartman JE, Ten Hacken NH, Klooster K, Slebos D-J. The minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPD. European Respiratory Journal 2015;46:1598-604. - PubMed
WHO 2003
-
- World Health Organization. Adherence to long-term therapies: evidence for action. apps.who.int/medicinedocs/pdf/s4883e/s4883e.pdf (accessed prior to 5 March 2019).
WHO 2017
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD). www.who.int/en/news-room/fact-sheets/detail/chronic-obstructive-pulmonar... (accessed prior to 29 March 2019).
Wijkstra 1994
Wiśniewski 2014
-
- Wiśniewski D, Porzezińska M, Gruchała-Niedoszytko M, Niedoszytko M, Słomiński JM, Jassem E. Factors influencing adherence to treatment in COPD patients and its relationship with disease exacerbations. Pneumonologia i Alergologia Polska 2014;82:96-104. - PubMed
Zhong 2014
-
- Zhong H, Ni X-J, Cui M, Liu X-Y. Evaluation of pharmacist care for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. International Journal of Clinical Pharmacy 2014;36(6):1230-40. - PubMed
References to other published versions of this review
Janjua 2019
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

