Physiologically Based Pharmacokinetic Modelling to Investigate the Impact of the Cytokine Storm on CYP3A Drug Pharmacokinetics in COVID-19 Patients
- PMID: 34496043
- PMCID: PMC8652944
- DOI: 10.1002/cpt.2402
Physiologically Based Pharmacokinetic Modelling to Investigate the Impact of the Cytokine Storm on CYP3A Drug Pharmacokinetics in COVID-19 Patients
Abstract
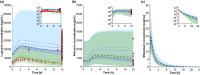
Patients with coronavirus disease 2019 (COVID-19) may experience a cytokine storm with elevated interleukin-6 (IL-6) levels in response to severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). IL-6 suppresses hepatic enzymes, including CYP3A; however, the effect on drug exposure and drug-drug interaction magnitudes of the cytokine storm and resulting elevated IL-6 levels have not been characterized in patients with COVID-19. We used physiologically-based pharmacokinetic (PBPK) modeling to simulate the effect of inflammation on the pharmacokinetics of CYP3A metabolized drugs. A PBPK model was developed for lopinavir boosted with ritonavir (LPV/r), using clinically observed data from people living with HIV (PLWH). The inhibition of CYPs by IL-6 was implemented by a semimechanistic suppression model and verified against clinical data from patients with COVID-19, treated with LPV/r. Subsequently, the verified model was used to simulate the effect of various clinically observed IL-6 levels on the exposure of LPV/r and midazolam, a CYP3A model drug. Clinically observed LPV/r concentrations in PLWH and patients with COVID-19 were predicted within the 95% confidence interval of the simulation results, demonstrating its predictive capability. Simulations indicated a twofold higher LPV exposure in patients with COVID-19 compared with PLWH, whereas ritonavir exposure was predicted to be comparable. Varying IL-6 levels under COVID-19 had only a marginal effect on LPV/r pharmacokinetics according to our model. Simulations showed that a cytokine storm increased the exposure of the CYP3A paradigm substrate midazolam by 40%. Our simulations suggest that CYP3A metabolism is altered in patients with COVID-19 having increased cytokine release. Caution is required when prescribing narrow therapeutic index drugs particularly in the presence of strong CYP3A inhibitors.
© 2021 The Authors. Clinical Pharmacology & Therapeutics © 2021 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Dickmann, L.J. , Patel, S.K. , Rock, D.A. , Wienkers, L.C. & Slatter, J.G. Effects of interleukin‐6 (IL‐6) and an anti‐IL‐6 monoclonal antibody on drug‐metabolizing enzymes in human hepatocyte culture. Drug Metab. Dispos. 39, 1415–1422 (2011). - PubMed
-
- Schmitt, C. , Kuhn, B. , Zhang, X. , Kivitz, A. & Grange, S. Disease–drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin. Pharmacol. Ther. 89, 735–740 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

