Isoxazole-9 reduces enhanced fear responses and retrieval in ethanol-dependent male rats
- PMID: 34496069
- PMCID: PMC10112848
- DOI: 10.1002/jnr.24932
Isoxazole-9 reduces enhanced fear responses and retrieval in ethanol-dependent male rats
Abstract
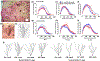
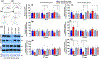
Plasticity in the dentate gyrus (DG) is strongly influenced by ethanol, and ethanol experience alters long-term memory consolidation dependent on the DG. However, it is unclear if DG plasticity plays a role in dysregulation of long-term memory consolidation during abstinence from chronic ethanol experience. Outbred male Wistar rats experienced 7 weeks of chronic intermittent ethanol vapor exposure (CIE). Seventy-two hours after CIE cessation, CIE and age-matched ethanol-naïve Air controls experienced auditory trace fear conditioning (TFC). Rats were tested for cue-mediated retrieval in the fear context either twenty-four hours (24 hr), ten days (10 days), or twenty-one days (21 days) later. CIE rats showed enhanced freezing behavior during TFC acquisition compared to Air rats. Air rats showed significant fear retrieval, and this behavior did not differ at the three time points. In CIE rats, fear retrieval increased over time during abstinence, indicating an incubation in fear responses. Enhanced retrieval at 21 days was associated with reduced structural and functional plasticity of ventral granule cell neurons (GCNs) and reduced expression of synaptic proteins important for neuronal plasticity. Systemic treatment with the drug Isoxazole-9 (Isx-9; small molecule that stimulates DG plasticity) during the last week and a half of CIE blocked altered acquisition and retrieval of fear memories in CIE rats during abstinence. Concurrently, Isx-9 modulated the structural and functional plasticity of ventral GCNs and the expression of synaptic proteins in the ventral DG. These findings identify that abstinence-induced disruption of fear memory consolidation occurs via altered plasticity within the ventral DG, and that Isx-9 prevented these effects.
Keywords: CIE; CaMKII; Fos; Golgi-Cox; excitability; trace fear conditioning.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures



References
-
- American Psychiatric Association, A.P.A.D.S.M.T.F. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Author.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

