What is the Sugar Code?
- PMID: 34496130
- PMCID: PMC8901795
- DOI: 10.1002/cbic.202100327
What is the Sugar Code?
Abstract
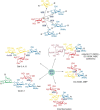
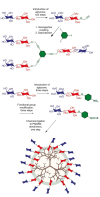
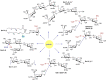
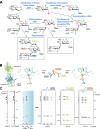
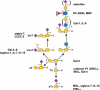
A code is defined by the nature of the symbols, which are used to generate information-storing combinations (e. g. oligo- and polymers). Like nucleic acids and proteins, oligo- and polysaccharides are ubiquitous, and they are a biochemical platform for establishing molecular messages. Of note, the letters of the sugar code system (third alphabet of life) excel in coding capacity by making an unsurpassed versatility for isomer (code word) formation possible by variability in anomery and linkage position of the glycosidic bond, ring size and branching. The enzymatic machinery for glycan biosynthesis (writers) realizes this enormous potential for building a large vocabulary. It includes possibilities for dynamic editing/erasing as known from nucleic acids and proteins. Matching the glycome diversity, a large panel of sugar receptors (lectins) has developed based on more than a dozen folds. Lectins 'read' the glycan-encoded information. Hydrogen/coordination bonding and ionic pairing together with stacking and C-H/π-interactions as well as modes of spatial glycan presentation underlie the selectivity and specificity of glycan-lectin recognition. Modular design of lectins together with glycan display and the nature of the cognate glycoconjugate account for the large number of post-binding events. They give an entry to the glycan vocabulary its functional, often context-dependent meaning(s), hereby building the dictionary of the sugar code.
Keywords: adhesion; glycoproteins; glycosylation; lectins; proliferation.
© 2021 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- N. Sharon, Complex Carbohydrates: Their Chemistry, Biosynthesis, and Functions, Addison-Wesley, Reading, 1975.
-
- Brandley B. K., Schnaar R. L., J. Leukocyte Biol. 1986, 40, 97–111. - PubMed
-
- Sharon N., Lis H., Science 1989, 246, 227–234. - PubMed
-
- Eichwald E., Justus Liebigs Ann. Chem.(Eur. J. Org.Chem.) 1865, 134, 177–211.
-
- None
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

