Development and Validation of a Stability-Indicating RP-HPLC Method for the Simultaneous Estimation of Bictegravir, Emtricitabine, and Tenofovir Alafenamide Fumarate
- PMID: 34496481
- PMCID: PMC8430401
- DOI: 10.4274/tjps.galenos.2020.70962
Development and Validation of a Stability-Indicating RP-HPLC Method for the Simultaneous Estimation of Bictegravir, Emtricitabine, and Tenofovir Alafenamide Fumarate
Abstract
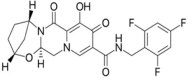
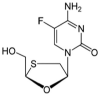
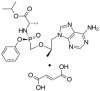
Objectives: The focal intent of the current research work is to develop and validate a novel and reliable stability-indicating reverse-phase high performance liquid chromatographic method for the simultaneous estimation of a few anti-retrovirals, i.e., bictegravir, emtricitabine, and tenofovir alafenamide fumarate (AF).
Materials and methods: The novel method employs inertsil octyldecylsilyl C18 (4.6×250 mm, 5 mm) using 0.2% triethylamine buffer and methanol in a ratio of 40:60% (v/v) as the mobile phase to attain optimal elution. The detection wavelength was 260 nm with a 1.2 mL/min flow rate and a 20 μL injection volume.
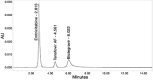
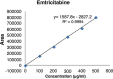
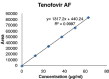
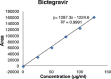
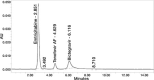
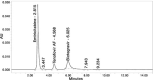
Results: The linearity ranges for bictegravir, emtricitabine and tenofovir AF were 25-125 μg/mL, 100-500 μg/mL, and 12.5-62.5 μg/mL, respectively. The retention times for bictegravir, emtricitabine, and tenofovir AF were found to be 5.998 min, 2.805 min, and 4.537, min respectively. The percent recoveries of bictegravir, emtricitabine, and tenofovir AF were within the range of 98-102% w/w.
Conclusion: The novel method was successfully validated as per International Conference on Harmonization guidelines. In forced degradation studies, emtricitabine was found to be sensitive to thermal conditions; bictegravir and tenofovir AF, to oxidative conditions. The developed method is economical and reliable for routine analysis concerning all validated parameters.
Keywords: Bictegravir; RP-HPLC; emtricitabine; forced degradation studies; tenofovir AF; validation.
Conflict of interest statement
Figures




Similar articles
-
Simultaneous determination of emtricitabine, tenofovir alafenamide fumarate and dolutegravir sodium by validated stability-indicating RP-HPLC-DAD method.Ann Pharm Fr. 2023 Jan;81(1):94-106. doi: 10.1016/j.pharma.2022.08.006. Epub 2022 Aug 28. Ann Pharm Fr. 2023. PMID: 36037931
-
Development And Validation Of Rp-Hplc Method For The Estimation Of Tenofovir And Emtricitabine In Bulk And Pharmaceutical Dosage Form.Curr Drug Res Rev. 2023 Jun 2. doi: 10.2174/2589977515666230602151222. Online ahead of print. Curr Drug Res Rev. 2023. PMID: 37278041
-
Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial.Lancet HIV. 2017 Apr;4(4):e154-e160. doi: 10.1016/S2352-3018(17)30016-4. Epub 2017 Feb 15. Lancet HIV. 2017. PMID: 28219610 Clinical Trial.
-
Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection.Drugs. 2018 Nov;78(17):1817-1828. doi: 10.1007/s40265-018-1010-7. Drugs. 2018. PMID: 30460547 Free PMC article. Review.
-
Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection.Drugs. 2016 Jun;76(9):957-68. doi: 10.1007/s40265-016-0586-z. Drugs. 2016. PMID: 27189707 Review.
Cited by
-
Simultaneous Quantification of Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate in Human Plasma by UPLC-MS/MS: Method Development and Validation.Turk J Pharm Sci. 2025 Aug 1;22(3):191-206. doi: 10.4274/tjps.galenos.2025.70718. Turk J Pharm Sci. 2025. PMID: 40746245 Free PMC article.
References
-
- Atkinson MJ, Petrozzino JJ. An evidence-based review of treatment-related determinants of patients’ nonadherence to HIV medications. AIDS Patient Care STDs. 2009;23:903–914. - PubMed
-
- Hester EK. HIV medications: an update and review of metabolic complications. Nutr Clin Pract. 2012;27:51–64. - PubMed
-
- Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, Tsai L, Bam RA, Stepan G, Stray KM, Niedziela-Majka A. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrobial Agents Chemother. 2016;60:7086–7097. - PMC - PubMed
LinkOut - more resources
Full Text Sources
