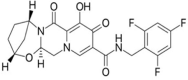
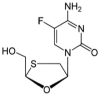
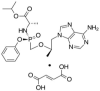
Development and Validation of a Stability-Indicating RP-HPLC Method for the Simultaneous Estimation of Bictegravir, Emtricitabine, and Tenofovir Alafenamide Fumarate
- PMID: 34496481
- PMCID: PMC8430401
- DOI: 10.4274/tjps.galenos.2020.70962
Development and Validation of a Stability-Indicating RP-HPLC Method for the Simultaneous Estimation of Bictegravir, Emtricitabine, and Tenofovir Alafenamide Fumarate
Abstract
Objectives: The focal intent of the current research work is to develop and validate a novel and reliable stability-indicating reverse-phase high performance liquid chromatographic method for the simultaneous estimation of a few anti-retrovirals, i.e., bictegravir, emtricitabine, and tenofovir alafenamide fumarate (AF).
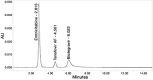
Materials and methods: The novel method employs inertsil octyldecylsilyl C18 (4.6×250 mm, 5 mm) using 0.2% triethylamine buffer and methanol in a ratio of 40:60% (v/v) as the mobile phase to attain optimal elution. The detection wavelength was 260 nm with a 1.2 mL/min flow rate and a 20 μL injection volume.
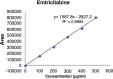
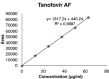
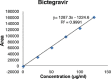
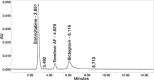
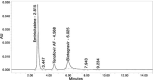
Results: The linearity ranges for bictegravir, emtricitabine and tenofovir AF were 25-125 μg/mL, 100-500 μg/mL, and 12.5-62.5 μg/mL, respectively. The retention times for bictegravir, emtricitabine, and tenofovir AF were found to be 5.998 min, 2.805 min, and 4.537, min respectively. The percent recoveries of bictegravir, emtricitabine, and tenofovir AF were within the range of 98-102% w/w.
Conclusion: The novel method was successfully validated as per International Conference on Harmonization guidelines. In forced degradation studies, emtricitabine was found to be sensitive to thermal conditions; bictegravir and tenofovir AF, to oxidative conditions. The developed method is economical and reliable for routine analysis concerning all validated parameters.
Keywords: Bictegravir; RP-HPLC; emtricitabine; forced degradation studies; tenofovir AF; validation.
Conflict of interest statement
Figures




References
-
- Atkinson MJ, Petrozzino JJ. An evidence-based review of treatment-related determinants of patients’ nonadherence to HIV medications. AIDS Patient Care STDs. 2009;23:903–914. - PubMed
-
- Hester EK. HIV medications: an update and review of metabolic complications. Nutr Clin Pract. 2012;27:51–64. - PubMed
-
- Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, Tsai L, Bam RA, Stepan G, Stray KM, Niedziela-Majka A. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrobial Agents Chemother. 2016;60:7086–7097. - PMC - PubMed
LinkOut - more resources
Full Text Sources
