Influence of Glucose Fluctuation on Peripheral Nerve Damage in Streptozotocin-Induced Diabetic Rats
- PMID: 34496549
- PMCID: PMC8831810
- DOI: 10.4093/dmj.2020.0275
Influence of Glucose Fluctuation on Peripheral Nerve Damage in Streptozotocin-Induced Diabetic Rats
Abstract
Background: It is unclear whether glycemic variability (GV) is a risk factor for diabetic peripheral neuropathy (DPN), and whether control of GV is beneficial for DPN. The purpose of this study was to investigate the effect of GV on peripheral nerve damage by inducing glucose fluctuation in streptozotocin-induced diabetic rats.
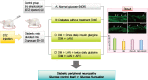
Methods: Rats were divided into four groups: normal (normal glucose group [NOR]), diabetes without treatment (sustained severe hyperglycemia group; diabetes mellitus [DM]), diabetes+once daily insulin glargine (stable hyperglycemia group; DM+LAN), and diabetes+once daily insulin glargine with twice daily insulin glulisine (unstable glucose fluctuation group; DM+Lantus [LAN]+Apidra [API]). We measured anti-oxidant enzyme levels and behavioral responses against tactile, thermal, and pressure stimuli in the plasma of rats. We also performed a quantitative comparison of cutaneous and sciatic nerves according to glucose fluctuation.
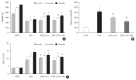
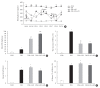
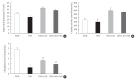
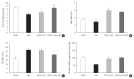
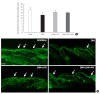
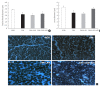
Results: At week 24, intraepidermal nerve fiber density was less reduced in the insulin-administered groups compared to the DM group (P<0.05); however, a significant difference was not observed between the DM+LAN and DM+LAN+API groups irrespective of glucose fluctuation (P>0.05; 16.2±1.6, 12.4±2.0, 14.3±0.9, and 13.9±0.6 for NOR, DM, DM+LAN, and DM+LAN+API, respectively). The DM group exhibited significantly decreased glutathione levels compared to the insulin-administered groups (2.64±0.10 μmol/mL, DM+LAN; 1.93±0.0 μmol/mL, DM+LAN+API vs. 1.25±0.04 μmol/mL, DM; P<0.05).
Conclusion: Our study suggests that glucose control itself is more important than glucose fluctuation in the prevention of peripheral nerve damage, and intra-day glucose fluctuation has a limited effect on the progression of peripheral neuropathy in rats with diabetes.
Keywords: Diabetes mellitus; Diabetic neuropathies; Insulin; Peripheral nerves.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–4. - PubMed
-
- Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (3rd and last part) Diabete Metab. 1977;3:245–56. - PubMed
-
- Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, et al. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia. 2009;52:1279–89. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

