Analytical Method Development and Validation for Simultaneous Determination of Simvastatin and Mupirocin Using Reverse-Phase High-pressure Liquid Chromatographic Method
- PMID: 34496550
- PMCID: PMC8430408
- DOI: 10.4274/tjps.galenos.2020.58897
Analytical Method Development and Validation for Simultaneous Determination of Simvastatin and Mupirocin Using Reverse-Phase High-pressure Liquid Chromatographic Method
Abstract
Objectives: This study was aimed to develop and validate the use of reverse-phase high pressure liquid chromatographic method for the estimation of simvastatin (SIM) and mupirocin (MUP) simultaneously.
Materials and methods: The chromatographic method developed is optimized for flow rate with the column, solvent, and buffer used and mobile phase ratio, molarity, and pH. The validation of the optimized method and the forced degradation studies of both drugs (under acidic, alkaline, oxidation, heat, light, and neutral conditions) were conducted following the The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines.
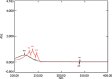
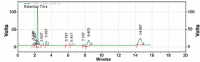
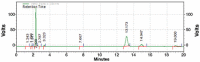
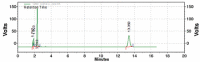
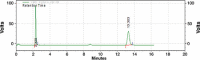
Results: Kromasil C18 column (250 mm × 4.6 mm, 5 µm) with ultraviolet detection at 224 nm and acetonitrile/phosphate buffer (30 mM, 70:30 v/v, pH 3.5; adjustment done using orthophosphoric acid) as mobile phase at a flow rate of 1.1 mL/min were observed to provide a good resolution for MUP and SIM at retention times of 2.32±0.008 and 13.55±0.254 min, respectively, with high accuracy (percent recovery was 99.69±0.82 for MUP and 101.10±0.02 for SIM) and linearity in the range of 5-30 µg/mL (r2: 0.9969 for MUP and r2: 0.9959 for SIM). The diagnostic limit and the lower limit of determination were 0.771±0.234 and 2.338±0.246 μg/mL for MUP and 0.595±0.282 and 1.803±0.334 µg/mL for SIM, respectively. The validated method was used to understand the degradation behavior of both drugs after the forced degradation studies.
Conclusion: The analytical method developed is determined to be specific, sensitive, precise, and accurate for the estimation of MUP and SIM simultaneously in the combined dosage form.
Keywords: Simvastatin; mupirocin; stability; validation.
Conflict of interest statement
Figures



References
-
- Thornton SC, Taylor SC, Weinberg JM. Topical antimicrobial agents in dermatology. Clin Dermatol. 2003;21:70–77. - PubMed
LinkOut - more resources
Full Text Sources
