Blood fibrocytes are associated with severity and prognosis in COVID-19 pneumonia
- PMID: 34496650
- PMCID: PMC8562948
- DOI: 10.1152/ajplung.00105.2021
Blood fibrocytes are associated with severity and prognosis in COVID-19 pneumonia
Abstract
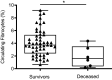
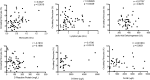
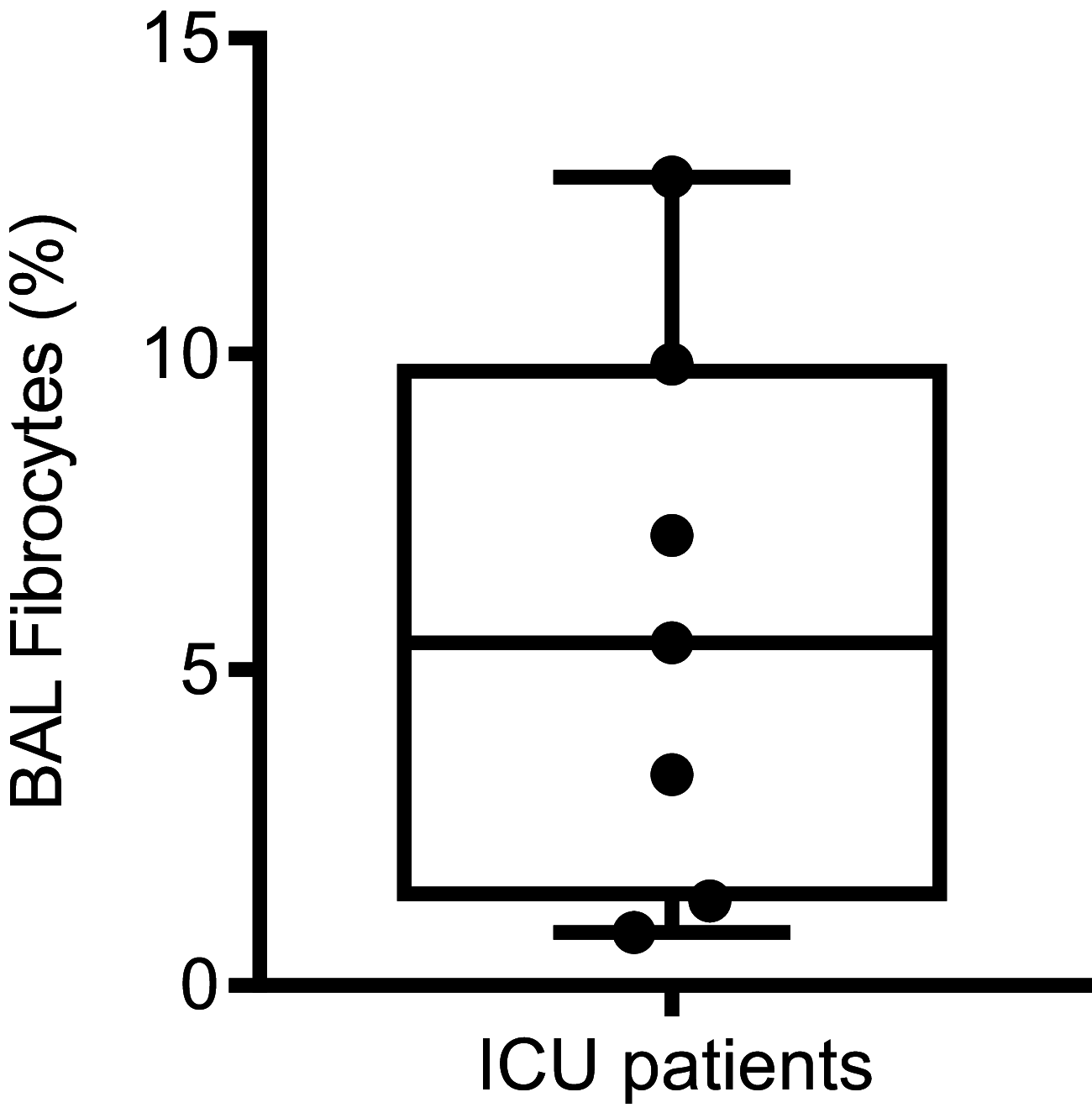
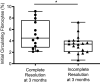
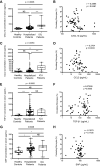
Increased blood fibrocytes are associated with a poor prognosis in fibrotic lung diseases. We aimed to determine whether the percentage of circulating fibrocytes could be predictive of severity and prognosis during coronavirus disease 2019 (COVID-19) pneumonia. Blood fibrocytes were quantified by flow cytometry as CD45+/CD15-/CD34+/collagen-1+ cells in patients hospitalized for COVID-19 pneumonia. In a subgroup of patients admitted in an intensive care unit (ICU), fibrocytes were quantified in blood and bronchoalveolar lavage (BAL). Serum amyloid P (SAP), transforming growth factor-β1 (TGF-β1), CXCL12, CCL2, and FGF2 concentrations were measured. We included 57 patients in the hospitalized group (median age = 59 yr [23-87]) and 16 individuals as healthy controls. The median percentage of circulating fibrocytes was higher in the patients compared with the controls (3.6% [0.2-9.2] vs. 2.1% [0.9-5.1], P = 0.04). Blood fibrocyte count was lower in the six patients who died compared with the survivors (1.6% [0.2-4.4] vs. 3.7% [0.6-9.2], P = 0.02). Initial fibrocyte count was higher in patients showing a complete lung computed tomography (CT) resolution at 3 mo. Circulating fibrocyte count was decreased in the ICU group (0.8% [0.1-2.0]), whereas BAL fibrocyte count was 6.7% (2.2-15.4). Serum SAP and TGF-β1 concentrations were increased in hospitalized patients. SAP was also increased in ICU patients. CXCL12 and CCL2 were increased in ICU patients and negatively correlated with circulating fibrocyte count. We conclude that circulating fibrocytes were increased in patients hospitalized for COVID-19 pneumonia, and a lower fibrocyte count was associated with an increased risk of death and a slower resolution of lung CT opacities.
Keywords: COVID-19 pneumonia; fibrocyte; prognosis.
Conflict of interest statement
B. Crestani is the principal investigator of the NINTECOR trial (NCT04541680) (Nintedanib for the Treatment of SARS-Cov-2 Induced Pulmonary Fibrosis). None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures


References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, Sinn BV, Gerhold M, Hauptmann K, Ingold-Heppner B, Miller F, Herbst H, Corman VM, Martin H, Radbruch H, Heppner FL, Horst D. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep 11: 4263, 2021. doi: 10.1038/s41598-021-82862-5. - DOI - PMC - PubMed
-
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands J, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, Voort PH, Mulder DJ, Goor H. Angiotensin‐converting enzyme 2 (ACE2), SARS‐CoV‐2 and the pathophysiology of coronavirus disease 2019 (COVID‐19). J Pathol 251: 228–248, 2020. doi: 10.1002/path.5471. - DOI - PMC - PubMed
-
- Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, Vinarsky V, Rubin J, Okin DA, Sclafani A, Alladina JW, Griffith JW, Gillette MA, Raz Y, Richards CJ, Wong AK, Ly A, Hung YP, Chivukula RR, Petri CR, Calhoun TF, Brenner LN, Hibbert KA, Medoff BD, Hardin CC, Stone JR, Mino-Kenudson M. Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory syndrome and H1N1 influenza: a systematic review. Chest 159: 73–84, 2021. doi: 10.1016/j.chest.2020.09.259. - DOI - PMC - PubMed
-
- Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. medRxiv 2020.04.06.20050575, 2020. doi: 10.1101/2020.04.06.20050575. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

