Regulation of wound ethylene biosynthesis by NAC transcription factors in kiwifruit
- PMID: 34496770
- PMCID: PMC8425125
- DOI: 10.1186/s12870-021-03154-8
Regulation of wound ethylene biosynthesis by NAC transcription factors in kiwifruit
Abstract
Background: The phytohormone ethylene controls many processes in plant development and acts as a key signaling molecule in response to biotic and abiotic stresses: it is rapidly induced by flooding, wounding, drought, and pathogen attack as well as during abscission and fruit ripening. In kiwifruit (Actinidia spp.), fruit ripening is characterized by two distinct phases: an early phase of system-1 ethylene biosynthesis characterized by absence of autocatalytic ethylene, followed by a late burst of autocatalytic (system-2) ethylene accompanied by aroma production and further ripening. Progress has been made in understanding the transcriptional regulation of kiwifruit fruit ripening but the regulation of system-1 ethylene biosynthesis remains largely unknown. The aim of this work is to better understand the transcriptional regulation of both systems of ethylene biosynthesis in contrasting kiwifruit organs: fruit and leaves.
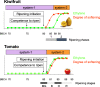
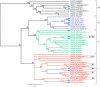
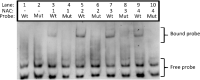
Results: A detailed molecular study in kiwifruit (A. chinensis) revealed that ethylene biosynthesis was regulated differently between leaf and fruit after mechanical wounding. In fruit, wound ethylene biosynthesis was accompanied by transcriptional increases in 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS), ACC oxidase (ACO) and members of the NAC class of transcription factors (TFs). However, in kiwifruit leaves, wound-specific transcriptional increases were largely absent, despite a more rapid induction of ethylene production compared to fruit, suggesting that post-transcriptional control mechanisms in kiwifruit leaves are more important. One ACS member, AcACS1, appears to fulfil a dominant double role; controlling both fruit wound (system-1) and autocatalytic ripening (system-2) ethylene biosynthesis. In kiwifruit, transcriptional regulation of both system-1 and -2 ethylene in fruit appears to be controlled by temporal up-regulation of four NAC (NAM, ATAF1/2, CUC2) TFs (AcNAC1-4) that induce AcACS1 expression by directly binding to the AcACS1 promoter as shown using gel-shift (EMSA) and by activation of the AcACS1 promoter in planta as shown by gene activation assays combined with promoter deletion analysis.
Conclusions: Our results indicate that in kiwifruit the NAC TFs AcNAC2-4 regulate both system-1 and -2 ethylene biosynthesis in fruit during wounding and ripening through control of AcACS1 expression levels but not in leaves where post-transcriptional/translational regulatory mechanisms may prevail.
Keywords: Biosynthesis; Ethylene; Kiwifruit; NAC; Regulation; Transcription factors; Wounding.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Morgan PW, Drew MC. Ethylene and plant responses to stress. Physiol Plantarum. 1997;100(3):620–630. doi: 10.1111/j.1399-3054.1997.tb03068.x. - DOI
-
- Pierik R, Sasidharan R, Voesenek LACJ. Growth control by ethylene: adjusting phenotypes to the environment. J Plant Growth Regul. 2007;26(2):188–200. doi: 10.1007/s00344-006-0124-4. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

