Bufalin suppresses tumour microenvironment-mediated angiogenesis by inhibiting the STAT3 signalling pathway
- PMID: 34496870
- PMCID: PMC8424978
- DOI: 10.1186/s12967-021-03058-z
Bufalin suppresses tumour microenvironment-mediated angiogenesis by inhibiting the STAT3 signalling pathway
Abstract
Background: Antiangiogenic therapy has increasingly become an important strategy for the treatment of colorectal cancer. Recent studies have shown that the tumour microenvironment (TME) promotes tumour angiogenesis. Bufalin is an active antitumour compound whose efficacy has been indicated by previous studies. However, there are very few studies on the antiangiogenic effects of bufalin.
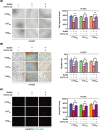
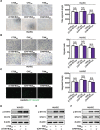
Methods: Herein, human umbilical vein endothelial cell (HUVEC) tube formation, migration and adhesion tests were used to assess angiogenesis in vitro. Western blotting and quantitative PCR were used to detect relevant protein levels and mRNA expression levels. A subcutaneous xenograft tumour model and a hepatic metastasis model were established in mice to investigate the influence of bufalin on angiogenesis mediated by the TME in vivo.
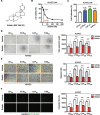
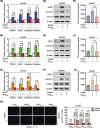
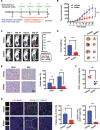
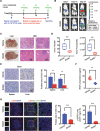
Results: We found that angiogenesis mediated by cells in the TME was significantly inhibited in the presence of bufalin. The results demonstrated that the proangiogenic genes in HUVECs, such as VEGF, PDGFA, E-selectin and P-selectin, were downregulated by bufalin and that this downregulation was mediated by inhibition of the STAT3 pathway. Overexpression of STAT3 reversed the inhibitory effects of bufalin on angiogenesis. Furthermore, there was little reduction in angiogenesis when bufalin directly acted on the cells in the tumour microenvironment.
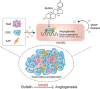
Conclusion: Our findings demonstrate that bufalin suppresses tumour microenvironment-mediated angiogenesis by inhibiting the STAT3 signalling pathway in vascular endothelial cells, revealing that bufalin may be used as a new antiangiogenic adjuvant therapy medicine to treat colorectal cancer.
Keywords: Angiogenesis; Bufalin; Colorectal cancer; STAT3; Tumour microenvironment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Synergistic anti-hepatoma effect of bufalin combined with sorafenib via mediating the tumor vascular microenvironment by targeting mTOR/VEGF signaling.Int J Oncol. 2018 Jun;52(6):2051-2060. doi: 10.3892/ijo.2018.4351. Epub 2018 Apr 2. Int J Oncol. 2018. PMID: 29620259
-
Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor-2.Br J Pharmacol. 2012 Feb;165(4b):1084-96. doi: 10.1111/j.1476-5381.2011.01532.x. Br J Pharmacol. 2012. PMID: 21658027 Free PMC article.
-
MicroRNA-497 and bufalin act synergistically to inhibit colorectal cancer metastasis.Tumour Biol. 2014 Mar;35(3):2599-606. doi: 10.1007/s13277-013-1342-6. Epub 2013 Dec 29. Tumour Biol. 2014. PMID: 24375248
-
Angiogenesis and targeted therapy in the tumour microenvironment: From basic to clinical practice.Clin Transl Med. 2025 Apr;15(4):e70313. doi: 10.1002/ctm2.70313. Clin Transl Med. 2025. PMID: 40268524 Free PMC article. Review.
-
The role of the tumour microenvironment in lung cancer and its therapeutic implications.Med Oncol. 2025 May 23;42(6):219. doi: 10.1007/s12032-025-02765-7. Med Oncol. 2025. PMID: 40407951 Free PMC article. Review.
Cited by
-
Roles of STAT3 in the pathogenesis and treatment of glioblastoma.Front Cell Dev Biol. 2023 Feb 27;11:1098482. doi: 10.3389/fcell.2023.1098482. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36923251 Free PMC article. Review.
-
Endothelial Stat3 activation promotes osteoarthritis development.Cell Prolif. 2023 Dec;56(12):e13518. doi: 10.1111/cpr.13518. Epub 2023 Jun 13. Cell Prolif. 2023. PMID: 37309689 Free PMC article.
-
Amphibian-Derived Natural Anticancer Peptides and Proteins: Mechanism of Action, Application Strategies, and Prospects.Int J Mol Sci. 2023 Sep 12;24(18):13985. doi: 10.3390/ijms241813985. Int J Mol Sci. 2023. PMID: 37762285 Free PMC article. Review.
-
Bufalin targeting BFAR inhibits the occurrence and metastasis of gastric cancer through PI3K/AKT/mTOR signal pathway.Apoptosis. 2023 Oct;28(9-10):1390-1405. doi: 10.1007/s10495-023-01855-z. Epub 2023 May 30. Apoptosis. 2023. PMID: 37253905
-
Patient-derived xenograft model in colorectal cancer basic and translational research.Animal Model Exp Med. 2023 Feb;6(1):26-40. doi: 10.1002/ame2.12299. Epub 2022 Dec 21. Animal Model Exp Med. 2023. PMID: 36543756 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ptkwws201905/Science and technology innovation project of Putuo District Health system
- 2019LK039/Budget project of Shanghai University of Traditional Chinese Medicine
- 20ZR1450500/Natural Science Foundation of Shanghai
- 19ZR1413800/Natural Science Foundation of Shanghai
- 81873137/National Nature Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

