Irisin alleviates obesity-related spermatogenesis dysfunction via the regulation of the AMPKα signalling pathway
- PMID: 34496874
- PMCID: PMC8424900
- DOI: 10.1186/s12958-021-00821-1
Irisin alleviates obesity-related spermatogenesis dysfunction via the regulation of the AMPKα signalling pathway
Abstract
Background: Infertility is a common complication in obese men. Oxidative stress and testicular apoptosis play critical roles in obesity-induced spermatogenesis dysfunction. It has been reported that irisin, an exercise-induced myokine, may attenuate oxidative damage and testicular apoptosis in several diseases; however, its role in obesity-induced spermatogenesis dysfunction remains unclear. The purpose of this study was to investigate the role and underlying mechanism of irisin in obesity-induced dysfunction of spermatogenesis.
Methods: Male mice were fed a high-fat diet (HFD) for 24 weeks to establish a model of obesity-induced spermatogenesis dysfunction. To explore the effects of irisin, mice were subcutaneously infused with recombinant irisin for 8 weeks beginning at 16 weeks after starting a HFD. To confirm the role of AMP-activated protein kinase α (AMPKα), AMPKα-deficient mice were used.
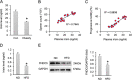
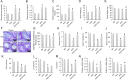
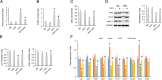
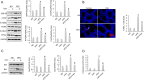
Results: The data showed decreased serum irisin levels in obese patients, which was negatively correlated with sperm count and progressive motility. Irisin was downregulated in the plasma and testes of obese mice. Supplementation with irisin protected against HFD-induced spermatogenesis dysfunction and increased testosterone levels in mice. HFD-induced oxidative stress, endoplasmic reticulum (ER) stress and testicular apoptosis were largely attenuated by irisin treatment. Mechanistically, we identified that irisin activated the AMPKα signalling pathway. With AMPKα depletion, we found that the protective effects of irisin on spermatogenesis dysfunction were abolished in vivo and in vitro.
Conclusions: In conclusion, we found that irisin alleviated obesity-related spermatogenesis dysfunction via activation of the AMPKα signalling pathway. Based on these findings, we hypothesized that irisin is a potential therapeutic agent against obesity-related spermatogenesis dysfunction.
Keywords: AMPKα; HFD; Irisin; Spermatogenesis dysfunction.
© 2021. The Author(s).
Conflict of interest statement
None declared.
Figures




References
-
- Best D, Bhattacharya S. Obesity and fertility. Horm Mol Biol Clin Investig. 2015;24(1):5–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

