Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury
- PMID: 34496892
- PMCID: PMC8425042
- DOI: 10.1186/s12951-021-01022-z
Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury
Abstract
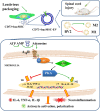
Background: Spinal cord injury (SCI) is an inflammatory condition, and excessive adenosine triphosphate (ATP) is released into the extracellular space, which can be catabolized into adenosine by CD73. Extracellular vesicles have been designed as nano drug carriers in many diseases. However, their impacts on delivery of CD73 after SCI are not yet known. We aimed to construct CD73 modified extracellular vesicles and explore the anti-inflammatory effects after SCI.
Methods: CD73 engineered extracellular vesicles (CD73+ hucMSC-EVs) were firstly established, which were derived from human umbilical cord mesenchymal stem cells (hucMSCs) transduced by lentiviral vectors to upregulate the expression of CD73. Effects of CD73+ hucMSC-EVs on hydrolyzing ATP into adenosine were detected. The polarization of M2/M1 was verified by immunofluorescence. Furthermore, A2aR and A2bR inhibitors and A2bR knockdown cells were used to investigate the activated adenosine receptor. Biomarkers of microglia and levels of cAMP/PKA were also detected. Repetitively in vivo study, morphology staining, flow cytometry, cytokine analysis, and ELISA assay, were also applied for verifications.
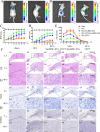
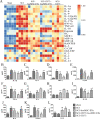
Results: CD73+ hucMSC-EVs reduced concentration of ATP and promoted the level of adenosine. In vitro experiments, CD73+ hucMSC-EVs increased macrophages/microglia M2:M1 polarization, activated adenosine 2b receptor (A2bR), and then promoted cAMP/PKA signaling pathway. In mice using model of thoracic spinal cord contusion injury, CD73+ hucMSC-EVs improved the functional recovery after SCI through decreasing the content of ATP in cerebrospinal fluid and improving the polarization from M1 to M2 phenotype. Thus, the cascaded pro-inflammatory cytokines were downregulated, such as TNF-α, IL-1β, and IL-6, while the anti-inflammatory cytokines were upregulated, such as IL-10 and IL-4.
Conclusions: CD73+ hucMSC-EVs ameliorated inflammation after spinal cord injury by reducing extracellular ATP, promoting A2bR/cAMP/PKA pathway and M2/M1 polarization. CD73+ hucMSC-EVs might be promising nano drugs for clinical application in SCI therapy.
Keywords: CD73; Extracellular vesicles; Inflammation; Mesenchymal stem cell; Spinal cord injury.
© 2021. The Author(s).
Conflict of interest statement
The authors do not have any possible conflicts of interests.
Figures





Similar articles
-
IL-4-primed human umbilical cord mesenchymal stem cells-derived extracellular vesicles facilitate recovery in spinal cord injury via the miR-21-5p/PDCD4-mediated shifting of macrophage M1/M2 polarization.Life Sci. 2025 Mar 1;364:123441. doi: 10.1016/j.lfs.2025.123441. Epub 2025 Feb 3. Life Sci. 2025. PMID: 39909387
-
Neuroprotective mechanism of human umbilical cord mesenchymal stem cell-derived extracellular vesicles improving the phenotype polarization of microglia via the PI3K/AKT/Nrf2 pathway in vascular dementia.Synapse. 2023 Jul;77(4):e22268. doi: 10.1002/syn.22268. Epub 2023 Apr 13. Synapse. 2023. PMID: 36941024
-
Ecto-5'-nucleotidase (CD73) attenuates inflammation after spinal cord injury by promoting macrophages/microglia M2 polarization in mice.J Neuroinflammation. 2018 May 22;15(1):155. doi: 10.1186/s12974-018-1183-8. J Neuroinflammation. 2018. PMID: 29788960 Free PMC article.
-
Therapeutic Efficacy and Promise of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles in Aging and Age-Related Disorders.Int J Mol Sci. 2024 Dec 30;26(1):225. doi: 10.3390/ijms26010225. Int J Mol Sci. 2024. PMID: 39796081 Free PMC article. Review.
-
Mesenchymal Stem Cells and Their Extracellular Vesicles: Therapeutic Mechanisms for Blood-Spinal Cord Barrier Repair Following Spinal Cord Injury.Int J Mol Sci. 2024 Dec 16;25(24):13460. doi: 10.3390/ijms252413460. Int J Mol Sci. 2024. PMID: 39769223 Free PMC article. Review.
Cited by
-
Mesenchymal stem cell-derived extracellular vesicles: emerging concepts in the treatment of spinal cord injury.Am J Transl Res. 2023 Jul 15;15(7):4425-4438. eCollection 2023. Am J Transl Res. 2023. PMID: 37560238 Free PMC article. Review.
-
Yin-Yang: two sides of extracellular vesicles in inflammatory diseases.J Nanobiotechnology. 2024 Aug 27;22(1):514. doi: 10.1186/s12951-024-02779-9. J Nanobiotechnology. 2024. PMID: 39192300 Free PMC article. Review.
-
Hydrogel-encapsulated extracellular vesicles for the regeneration of spinal cord injury.Front Neurosci. 2023 Dec 14;17:1309172. doi: 10.3389/fnins.2023.1309172. eCollection 2023. Front Neurosci. 2023. PMID: 38156267 Free PMC article. Review.
-
P2X7 receptor activation awakes a dormant stem cell niche in the adult spinal cord.Front Cell Neurosci. 2023 Dec 18;17:1288676. doi: 10.3389/fncel.2023.1288676. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38164435 Free PMC article.
-
MiR-30d-5p Modulates Glucose and Lipid Metabolism by Targeting CD73 through the AMPK Pathway.Cell Biochem Biophys. 2025 Jul 5. doi: 10.1007/s12013-025-01815-1. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40618010
References
-
- Dodiya HB, Kuntz T, Shaik SM, Baufeld C, Leibowitz J, Zhang X, Gottel N, Zhang X, Butovsky O, Gilbert JA, Sisodia SS. Sex-specific effects of microbiome perturbations on cerebral Abeta amyloidosis and microglia phenotypes. J Exp Med. 2019;216:1542–1560. doi: 10.1084/jem.20182386. - DOI - PMC - PubMed
-
- Gotzl JK, Brendel M, Werner G, Parhizkar S, Sebastian Monasor L, Kleinberger G, Colombo AV, Deussing M, Wagner M, Winkelmann J, Diehl-Schmid J, Levin J, Fellerer K, Reifschneider A, Bultmann S, Bartenstein P, Rominger A, Tahirovic S, Smith ST, Madore C, Butovsky O, Capell A, Haass C. Opposite microglial activation stages upon loss of PGRN or TREM2 result in reduced cerebral glucose metabolism. EMBO Mol Med. 2019;11(6):e9711. doi: 10.15252/emmm.201809711. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 81701199/National Natural Science Foundation of China
- ZY[2018-2020]-FWTX-2005/State Administration of Traditional Chinese Medicine of the People's Republic of China
- 17441900500/Science and Technology Commission of Shanghai Municipality
- 16DZ0504000/Science and Technology Commission of Shanghai Municipality
LinkOut - more resources
Full Text Sources
Medical
Research Materials

