Antimicrobial peptides: mechanism of action, activity and clinical potential
- PMID: 34496967
- PMCID: PMC8425997
- DOI: 10.1186/s40779-021-00343-2
Antimicrobial peptides: mechanism of action, activity and clinical potential
Abstract
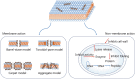
The management of bacterial infections is becoming a major clinical challenge due to the rapid evolution of antibiotic resistant bacteria. As an excellent candidate to overcome antibiotic resistance, antimicrobial peptides (AMPs) that are produced from the synthetic and natural sources demonstrate a broad-spectrum antimicrobial activity with the high specificity and low toxicity. These peptides possess distinctive structures and functions by employing sophisticated mechanisms of action. This comprehensive review provides a broad overview of AMPs from the origin, structural characteristics, mechanisms of action, biological activities to clinical applications. We finally discuss the strategies to optimize and develop AMP-based treatment as the potential antimicrobial and anticancer therapeutics.
Keywords: Antimicrobial peptides; Antimicrobial resistance; Biological activity; Clinical application; Mechanism of action.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

