Treatment patterns in people with cystic fibrosis: have they changed since the introduction of ivacaftor?
- PMID: 34497037
- PMCID: PMC9097695
- DOI: 10.1016/j.jcf.2021.08.014
Treatment patterns in people with cystic fibrosis: have they changed since the introduction of ivacaftor?
Abstract
Background: In late 2012, ivacaftor became available in the UK for people with cystic fibrosis (CF) aged 6 years and over with a G551D mutation. Long-term changes in treatment patterns have not previously been reported. We investigated long-term treatment patterns in people with CF with a G551D mutation who took ivacaftor and compared these with non-ivacaftor-treated cohorts using the UK Cystic Fibrosis Registry.
Methods: Using 2007-2018 data we compared treatment patterns between four cohorts: 1: ivacaftor-treated; 2: ivacaftor era (2013-2018), ineligible genotype (no G551D mutation); 3: pre-ivacaftor era (2007-2012), eligible genotype (G551D mutation); 4: pre-ivacaftor era, ineligible genotype. Treatments included: inhaled antibiotics, dornase alfa, hypertonic saline, chronic oral antibiotics and supplementary feeding.
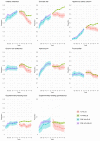
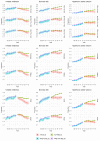
Results: Up to 2012 the percentages of people taking each treatment were similar between the two cohorts defined by genotype and tended to increase by year with a similar slope. Once ivacaftor was introduced, the use of other treatments tended to decrease or remain stable by year for the ivacaftor-treated cohort, whereas it remained stable or increased in the non-ivacaftor-treated cohort. This led to differences in treatment use between the two cohorts in the ivacaftor-era, which became more marked over time.
Conclusions: We have shown a clear divergence in treatment patterns since the introduction of ivacaftor in a number of key treatments widely used in CF. Further research is needed to investigate whether the differences in treatment patterns are associated with changes in health outcomes.
Keywords: cystic fibrosis; ivacaftor; registry data; treatment burden; treatment patterns.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest statement GD has received personal fees from Chiesi Limited for lectures, unrelated to the current work. She is co-Chief Investigator for the CF STORM clinical trial. EG has no conflicts of interest to declare. RHK received funding from a Circle of Care Award from Vertex.
Figures
Similar articles
-
Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data.Am J Respir Crit Care Med. 2015 Oct 1;192(7):836-42. doi: 10.1164/rccm.201503-0578OC. Am J Respir Crit Care Med. 2015. PMID: 26132840
-
[Real-world effectiveness of ivacaftor in children with cystic fibrosis and the G551D mutation].An Pediatr (Engl Ed). 2019 Mar;90(3):148-156. doi: 10.1016/j.anpedi.2018.05.022. Epub 2018 Aug 6. An Pediatr (Engl Ed). 2019. PMID: 30093322 Spanish.
-
Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2019 Jan 7;1(1):CD009841. doi: 10.1002/14651858.CD009841.pub3. Cochrane Database Syst Rev. 2019. PMID: 30616300 Free PMC article.
-
Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor.Clin Infect Dis. 2015 Mar 1;60(5):703-12. doi: 10.1093/cid/ciu944. Epub 2014 Nov 25. Clin Infect Dis. 2015. PMID: 25425629 Free PMC article.
-
Ivacaftor treatment of cystic fibrosis patients with the G551D mutation: a review of the evidence.Ther Adv Respir Dis. 2013 Oct;7(5):288-96. doi: 10.1177/1753465813502115. Epub 2013 Sep 3. Ther Adv Respir Dis. 2013. PMID: 24004658 Review.
Cited by
-
Exploring the impact of elexacaftor-tezacaftor-ivacaftor treatment on opinions regarding airway clearance techniques and nebulisers: TEMPO a qualitative study in children with cystic fibrosis, their families and healthcare professionals.BMJ Open Respir Res. 2022 Oct;9(1):e001420. doi: 10.1136/bmjresp-2022-001420. BMJ Open Respir Res. 2022. PMID: 36207030 Free PMC article.
-
Dornase alfa in Cystic Fibrosis: indications, comparative studies and effects on lung clearance index.Ital J Pediatr. 2022 Aug 4;48(1):141. doi: 10.1186/s13052-022-01331-5. Ital J Pediatr. 2022. PMID: 35927765 Free PMC article. Review.
-
Psychological interventions for improving adherence to inhaled therapies in people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Mar 29;3(3):CD013766. doi: 10.1002/14651858.CD013766.pub2. Cochrane Database Syst Rev. 2023. PMID: 36989170 Free PMC article. Review.
-
Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis: A Review of Registry-Based Evidence.J Clin Med. 2025 Jun 5;14(11):3978. doi: 10.3390/jcm14113978. J Clin Med. 2025. PMID: 40507740 Free PMC article. Review.
-
The CFHealthHub Learning Health System: Using Real-Time Adherence Data to Support a Community of Practice to Deliver Continuous Improvement in an Archetypal Long-Term Condition.Healthcare (Basel). 2022 Dec 21;11(1):20. doi: 10.3390/healthcare11010020. Healthcare (Basel). 2022. PMID: 36611480 Free PMC article. Review.
References
-
- Davies J., Sheridan H., Bell N., Cunningham S., Davis S.D., Elborn J.S. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1(8):630–638. - PubMed
-
- Sawicki G.S., McKone E.F., Pasta D.J., Millar S.J., Wagener J.S., Johnson C.A. Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192(7):836–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

