Regulatory and clinical consequences of negative confirmatory trials of accelerated approval cancer drugs: retrospective observational study
- PMID: 34497044
- PMCID: PMC8424519
- DOI: 10.1136/bmj.n1959
Regulatory and clinical consequences of negative confirmatory trials of accelerated approval cancer drugs: retrospective observational study
Abstract
Objectives: To investigate the regulatory handling of cancer drugs that were granted accelerated approval by the US Food and Drug Administration (FDA) but failed to improve the primary endpoint in post-approval trials and to evaluate the extent to which negative post-approval trials changed the recommendations in treatment guidelines.
Design: Retrospective observational study.
Setting: FDA and National Comprehensive Cancer Network (NCCN) reports.
Included drugs: Cancer drugs that received accelerated approval from the FDA and had negative post-approval trials.
Main outcome measures: Regulatory outcomes, including withdrawal, conversion to regular approval, and no action.
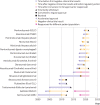
Results: 18 indications for 10 cancer drugs that received accelerated approval but failed to improve the primary endpoint in post-approval trials were identified. Of these, 11 (61%) were voluntarily withdrawn by the manufacturer and one (bevacizumab for breast cancer) was revoked by the FDA. Of the 11 withdrawals, six occurred in 2021 alone. The remaining six (33%) indications remain on the label. The NCCN guidelines provide a high level of endorsement (category 1 endorsement for one and category 2A endorsement for seven) for accelerated approval drugs that have failed post-approval trials, sometimes even after the approval has been withdrawn or revoked.
Conclusion: Cancer drug indications that received accelerated approval often remained on formal FDA approved drug labelling and continued to be recommended in clinical guidelines several years after statutorily required post-approval trials showed no improvement in the primary efficacy endpoint. Clinical guidelines should better align with the results of post-approval trials of cancer drugs that received accelerated approval.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support for the study from Arnold Ventures; ASK is principal investigator on a grant from the FDA to Brigham and Women’s Hospital on an unrelated topic; BG has received consulting fees from Vivio Health, outside the submitted work; BNR has received a grant from Anthem Public Policy Institute and personal fees from Blue Cross Blue Shield of Massachusetts, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Regulatory drug approval: protecting patients or serving impatient shareholders?BMJ. 2021 Sep 29;374:n2382. doi: 10.1136/bmj.n2382. BMJ. 2021. PMID: 34588181 No abstract available.
References
-
- FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource. US Food and Drug Administration, National Institutes of Health, 2016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
