Sulperazon-induced acute reactive thrombocytopenia during treatment of systemic lupus erythematosus: a case report
- PMID: 34497130
- PMCID: PMC10359788
- DOI: 10.1136/ejhpharm-2021-002999
Sulperazon-induced acute reactive thrombocytopenia during treatment of systemic lupus erythematosus: a case report
Abstract
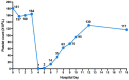
The purpose of this study is to report a patient who developed acute reactive thrombocytopenia while undergoing treatment with sulperazon for systemic lupus erythematosus (SLE). Sulperazon is a broad-spectrum antibiotic that can act against a wide range of microorganisms, but rarely causes severe thrombocytopenic events. We describe a 62-year-old man with new-onset acute reactive thrombocytopenia who experienced a precipitous fall in the platelet count from 168×109/L to 1×109/L within 29 hours after exposure to sulperazon. Sulperazon was immediately discontinued followed by administration of intravenous immunoglobulin for six consecutive days. The platelet count eventually recovered and petechiae at the injection sites improved. No complications secondary to acute reactive thrombocytopenia were observed except petechiae.
Keywords: case reports; clinical medicine; drug monitoring; education; pharmacy; pharmacy administration.
© European Association of Hospital Pharmacists 2023. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Cerebral Venous Sinus Thrombosis in Systemic Lupus Erythematosus.Acta Med Indones. 2018 Oct;50(4):343-345. Acta Med Indones. 2018. PMID: 30631001
-
Relapsed hydroxychloroquine induced thrombocytopenia in a systemic lupus erythematosus patient.Reumatol Clin. 2017 Sep-Oct;13(5):294-296. doi: 10.1016/j.reuma.2016.04.008. Epub 2016 Jun 2. Reumatol Clin. 2017. PMID: 27263963 English, Spanish.
-
Two cases of refractory thrombocytopenia in systemic lupus erythematosus that responded to intravenous low-dose cyclophosphamide.J Korean Med Sci. 2013 Mar;28(3):472-5. doi: 10.3346/jkms.2013.28.3.472. Epub 2013 Mar 4. J Korean Med Sci. 2013. PMID: 23487584 Free PMC article.
-
Response to belimumab in thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a case-based review.Clin Rheumatol. 2022 Aug;41(8):2561-2569. doi: 10.1007/s10067-022-06155-6. Epub 2022 May 7. Clin Rheumatol. 2022. PMID: 35524885 Review.
-
[A case of a thrombocytopenic crisis with a favorable outcome in a female patient with systemic lupus erythematosus].Lik Sprava. 1997 Sep-Oct;(5):179-81. Lik Sprava. 1997. PMID: 9491737 Review. Russian. No abstract available.
Cited by
-
Therapeutic efficacy of different sulperazon dosing regimens in carbapenem-resistant Klebsiella pneumoniae: a retrospective analysis of 209 cases.Am J Transl Res. 2025 Jun 15;17(6):4764-4773. doi: 10.62347/TZFB9853. eCollection 2025. Am J Transl Res. 2025. PMID: 40672637 Free PMC article.
References
-
- Suzuki K, Horiba M. [Laboratory and clinical study of sulbactam/cefoperazone (SBT/CPZ) on bacterial prostatitis]. Hinyokika Kiyo 1991;37:1333–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical