Toll-like receptor 1 as a possible target in non-alcoholic fatty liver disease
- PMID: 34497333
- PMCID: PMC8426394
- DOI: 10.1038/s41598-021-97346-9
Toll-like receptor 1 as a possible target in non-alcoholic fatty liver disease
Abstract
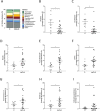
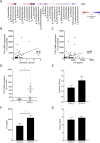
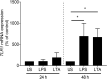
Toll-like receptors (TLRs) in the liver compartment have repeatedly been attributed to the development of non-alcoholic fatty liver disease (NAFLD). Knowledge on TLR expression in blood cells and their relation to intestinal microbiota and NAFLD development is limited. Here, we determined TLR expression patterns in peripheral blood mononuclear cells (PBMCs) of NAFLD patients and controls, their relation to intestinal microbiota and the impact of TLRs found altered in NAFLD development. Markers of intestinal permeability in blood and TLR mRNA expression in PBMCs were determined in 37 NAFLD patients and 15 age-matched healthy controls. Fecal microbiota composition was evaluated in 21 NAFLD patients and 9 controls using 16S rRNA gene amplicon sequencing. Furthermore, TLR1-/- and C57BL/6 mice (n = 5-6/group) were pair-fed a liquid control or a fat-, fructose- and cholesterol-rich diet. Intestinal microbiota composition and markers of intestinal permeability like zonulin and bacterial endotoxin differed significantly between groups with the latter markers being significantly higher in NAFLD patients. Expression of TLR1-8 and 10 mRNA was detectable in PBMCs; however, only TLR1 expression, being higher in NAFLD patients, were significantly positively correlated with the prevalence of Holdemanella genus while negative correlations were found with Gemmiger and Ruminococcus genera. TLR1-/- mice were significantly protected from the development of diet-induced NAFLD when compared to wild-type mice. While intestinal microbiota composition and permeability differed significantly between NAFLD patients and healthy subjects, in PBMCs, only TLR1 expression differed between groups. Still, targeting these alterations might be a beneficial approach in the treatment of NAFLD in some patients.
© 2021. The Author(s).
Conflict of interest statement
J.M.S. serves as a consultant or advisory board member for Boehringer Ingelheim, Bristol-Myer Squibb, Echosens, Gilead, Genfit, Intercept, IQVIA, Madrigal, Nordic Bioscience, Novartis, Novo Nordisk, Pfizer, Siemens Healthineers. In addition, his institution has received grant support from Gilead Sciences and Yakult Deutschland. I.B. received funding of Yakult. All other authors declare no conflict of interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

