Neutralizing antibodies for the prevention and treatment of COVID-19
- PMID: 34497376
- PMCID: PMC8424621
- DOI: 10.1038/s41423-021-00752-2
Neutralizing antibodies for the prevention and treatment of COVID-19
Abstract
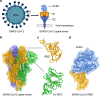
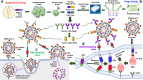
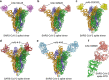
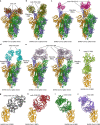
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) initiates the infection process by binding to the viral cellular receptor angiotensin-converting enzyme 2 through the receptor-binding domain (RBD) in the S1 subunit of the viral spike (S) protein. This event is followed by virus-cell membrane fusion mediated by the S2 subunit, which allows virus entry into the host cell. Therefore, the SARS-CoV-2 S protein is a key therapeutic target, and prevention and treatment of coronavirus disease 2019 (COVID-19) have focused on the development of neutralizing monoclonal antibodies (nAbs) that target this protein. In this review, we summarize the nAbs targeting SARS-CoV-2 proteins that have been developed to date, with a focus on the N-terminal domain and RBD of the S protein. We also describe the roles that binding affinity, neutralizing activity, and protection provided by these nAbs play in the prevention and treatment of COVID-19 and discuss the potential to improve nAb efficiency against multiple SARS-CoV-2 variants. This review provides important information for the development of effective nAbs with broad-spectrum activity against current and future SARS-CoV-2 strains.
Keywords: COVID-19; Monoclonal antibodies; Neutralization; SARS-CoV-2; Spike protein.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25:121. doi: 10.1186/s13054-021-03540-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI139092/AI/NIAID NIH HHS/United States
- R01 AI157975/AI/NIAID NIH HHS/United States
- R01 AI137472/AI/NIAID NIH HHS/United States
- R01AI157975/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AI137472/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

