Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia
- PMID: 34497422
- PMCID: PMC8711093
- DOI: 10.1038/s41586-021-03897-2
Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia
Abstract
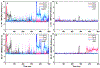
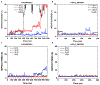
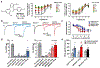
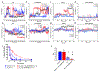
The adenosine A1 receptor (A1R) is a promising therapeutic target for non-opioid analgesic agents to treat neuropathic pain1,2. However, development of analgesic orthosteric A1R agonists has failed because of a lack of sufficient on-target selectivity as well as off-tissue adverse effects3. Here we show that [2-amino-4-(3,5-bis(trifluoromethyl)phenyl)thiophen-3-yl)(4-chlorophenyl)methanone] (MIPS521), a positive allosteric modulator of the A1R, exhibits analgesic efficacy in rats in vivo through modulation of the increased levels of endogenous adenosine that occur in the spinal cord of rats with neuropathic pain. We also report the structure of the A1R co-bound to adenosine, MIPS521 and a Gi2 heterotrimer, revealing an extrahelical lipid-detergent-facing allosteric binding pocket that involves transmembrane helixes 1, 6 and 7. Molecular dynamics simulations and ligand kinetic binding experiments support a mechanism whereby MIPS521 stabilizes the adenosine-receptor-G protein complex. This study provides proof of concept for structure-based allosteric drug design of non-opioid analgesic agents that are specific to disease contexts.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

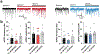


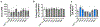



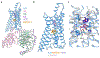

Comment in
-
Allosteric adenosine receptor modulator alleviates pain.Nat Rev Drug Discov. 2021 Nov;20(11):813. doi: 10.1038/d41573-021-00162-z. Nat Rev Drug Discov. 2021. PMID: 34561672 No abstract available.
References
-
- Nakamura I, Ohta Y, and Kemmotsu OM, Characterization of Adenosine Receptors Mediating Spinal Sensory Transmission Related to Nociceptive Information in the Rat. Anesthesiology, 1997. 87(3): p. 577–584. - PubMed
-
- Poon A and Sawynok J, Antinociception by adenosine analogs and inhibitors of adenosine metabolism in an inflammatory thermal hyperalgesia model in the rat. Pain, 1998. 74(2-3): p. 235–45. - PubMed
-
- King A, Analgesia without opioids. Nature, 2019. 573(7773): p. S4.
Method References
-
- Seltzer Z, Dubner R, and Shir Y, A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain, 1990. 43(2): p. 205–18. - PubMed
-
- Størkson RV, et al. , Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods, 1996. 65(2): p. 167–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

