Stress-activated kinases as therapeutic targets in pancreatic cancer
- PMID: 34497429
- PMCID: PMC8384741
- DOI: 10.3748/wjg.v27.i30.4963
Stress-activated kinases as therapeutic targets in pancreatic cancer
Abstract
Pancreatic cancer is a dismal disease with high incidence and poor survival rates. With the aim to improve overall survival of pancreatic cancer patients, new therapeutic approaches are urgently needed. Protein kinases are key regulatory players in basically all stages of development, maintaining physiologic functions but also being involved in pathogenic processes. c-Jun N-terminal kinases (JNK) and p38 kinases, representatives of the mitogen-activated protein kinases, as well as the casein kinase 1 (CK1) family of protein kinases are important mediators of adequate response to cellular stress following inflammatory and metabolic stressors, DNA damage, and others. In their physiologic roles, they are responsible for the regulation of cell cycle progression, cell proliferation and differentiation, and apoptosis. Dysregulation of the underlying pathways consequently has been identified in various cancer types, including pancreatic cancer. Pharmacological targeting of those pathways has been the field of interest for several years. While success in earlier studies was limited due to lacking specificity and off-target effects, more recent improvements in small molecule inhibitor design against stress-activated protein kinases and their use in combination therapies have shown promising in vitro results. Consequently, targeting of JNK, p38, and CK1 protein kinase family members may actually be of particular interest in the field of precision medicine in patients with highly deregulated kinase pathways related to these kinases. However, further studies are warranted, especially involving in vivo investigation and clinical trials, in order to advance inhibition of stress-activated kinases to the field of translational medicine.
Keywords: Casein kinase 1; Mitogen-activated protein kinases; Pancreatic cancer; Small molecule inhibitor; Stress-activated protein kinases; c-Jun N-terminal kinases.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
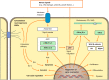
Figures
References
-
- Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology . 2015;15:8–18. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res . 2014;74:2913–2921. - PubMed
-
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev . 2006;20:1218–1249. - PubMed
-
- Turjanski AG, Vaqué JP, Gutkind JS. MAP kinases and the control of nuclear events. Oncogene . 2007;26:3240–3253. - PubMed
-
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res . 2002;12:9–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

