Estrogen augmented visceral pain and colonic neuron modulation in a double-hit model of prenatal and adult stress
- PMID: 34497435
- PMCID: PMC8384739
- DOI: 10.3748/wjg.v27.i30.5060
Estrogen augmented visceral pain and colonic neuron modulation in a double-hit model of prenatal and adult stress
Abstract
Background: Chronic stress during pregnancy may increase visceral hyperalgesia of offspring in a sex-dependent way. Combining adult stress in offspring will increase this sensitivity. Based on the evidence implicating estrogen in exacerbating visceral hypersensitivity in female rodents in preclinical models, we predicted that chronic prenatal stress (CPS) + chronic adult stress (CAS) will maximize visceral hyperalgesia; and that estrogen plays an important role in colonic hyperalgesia.
Aim: The aim was to illuminate the role of estrogen in colonic hyperalgesia and its underlying mechanisms.
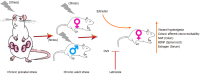
Methods: We established a CPS plus CAS rodent model in which the balloon was used to distend the colorectum. The single-fiber recording in vivo and patch clamp experiments in vitro were used to monitor the colonic neuron's activity. The reverse transcription-polymerase chain reaction, western blot, and immunofluorescence were used to study the effects of CPS and CAS on colon primary afferent sensitivity. We used ovariectomy and letrozole to reduce estrogen levels of female rats respectively in order to assess the role of estrogen in female-specific enhanced primary afferent sensitization.
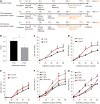
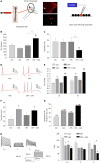
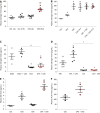
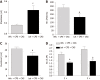
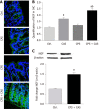
Results: Spontaneous activity and single fiber activity were significantly greater in females than in males. The enhanced sensitization in female rats mainly came from low-threshold neurons. CPS significantly increased single-unit afferent fiber activity in L6-S2 dorsal roots in response. Activity was further enhanced by CAS. In addition, the excitability of colon-projecting dorsal root ganglion (DRG) neurons increased in CPS + CAS rats and was associated with a decrease in transient A-type K+ currents. Compared with ovariectomy, treatment with the aromatase inhibitor letrozole significantly reduced estrogen levels in female rats, confirming the gender difference. Moreover, mice treated with letrozole had decreased colonic DRG neuron excitability. The intrathecal infusion of estrogen increased brain-derived neurotrophic factor (BDNF) protein levels and contributed to the response to visceral pain. Western blotting showed that nerve growth factor protein was upregulated in CPS + CAS mice.
Conclusion: This study adds to the evidence that estrogen-dependent sensitization of primary afferent colon neurons is involved in the development of chronic stress-induced visceral hypersensitivity in female rats.
Keywords: Chronic prenatal stress; Estrogen; Excitability; Letrozole; Neuronal sensitization; Visceral pain.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflicting interests.
Figures
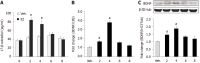
References
-
- Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. - PubMed
-
- Taub E, Cuevas JL, Cook EW 3rd, Crowell M, Whitehead WE. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Dig Dis Sci. 1995;40:2647–2655. - PubMed
-
- Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

