Contribution of Tissue Inflammation and Blood-Brain Barrier Disruption to Brain Softening in a Mouse Model of Multiple Sclerosis
- PMID: 34497486
- PMCID: PMC8419310
- DOI: 10.3389/fnins.2021.701308
Contribution of Tissue Inflammation and Blood-Brain Barrier Disruption to Brain Softening in a Mouse Model of Multiple Sclerosis
Abstract
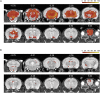
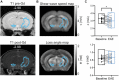
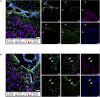
Neuroinflammatory processes occurring during multiple sclerosis cause disseminated softening of brain tissue, as quantified by in vivo magnetic resonance elastography (MRE). However, inflammation-mediated tissue alterations underlying the mechanical integrity of the brain remain unclear. We previously showed that blood-brain barrier (BBB) disruption visualized by MRI using gadolinium-based contrast agent (GBCA) does not correlate with tissue softening in active experimental autoimmune encephalomyelitis (EAE). However, it is unknown how confined BBB changes and other inflammatory processes may determine local elasticity changes. Therefore, we aim to elucidate which inflammatory hallmarks are determinant for local viscoelastic changes observed in EAE brains. Hence, novel multifrequency MRE was applied in combination with GBCA-based MRI or very small superparamagnetic iron oxide particles (VSOPs) in female SJL mice with induced adoptive transfer EAE (n = 21). VSOPs were doped with europium (Eu-VSOPs) to facilitate the post-mortem analysis. Accumulation of Eu-VSOPs, which was previously demonstrated to be sensitive to immune cell infiltration and ECM remodeling, was also found to be independent of GBCA enhancement. Following registration to a reference brain atlas, viscoelastic properties of the whole brain and areas visualized by either Gd or VSOP were quantified. MRE revealed marked disseminated softening across the whole brain in mice with established EAE (baseline: 3.1 ± 0.1 m/s vs. EAE: 2.9 ± 0.2 m/s, p < 0.0001). A similar degree of softening was observed in sites of GBCA enhancement i.e., mainly within cerebral cortex and brain stem (baseline: 3.3 ± 0.4 m/s vs. EAE: 3.0 ± 0.5 m/s, p = 0.018). However, locations in which only Eu-VSOP accumulated, mainly in fiber tracts (baseline: 3.0 ± 0.4 m/s vs. EAE: 2.6 ± 0.5 m/s, p = 0.023), softening was more pronounced when compared to non-hypointense areas (percent change of stiffness for Eu-VSOP accumulation: -16.81 ± 16.49% vs. for non-hypointense regions: -5.85 ± 3.81%, p = 0.048). Our findings suggest that multifrequency MRE is sensitive to differentiate between local inflammatory processes with a strong immune cell infiltrate that lead to VSOP accumulation, from disseminated inflammation and BBB leakage visualized by GBCA. These pathological events visualized by Eu-VSOP MRI and MRE may include gliosis, macrophage infiltration, alterations of endothelial matrix components, and/or extracellular matrix remodeling. MRE may therefore represent a promising imaging tool for non-invasive clinical assessment of different pathological aspects of neuroinflammation.
Keywords: BBB disruption; Eu-VSOP; experimental autoimmune encephalomyelitis; gadolinium; magnetic resonance elastography; multiple sclerosis; neuroinflammation.
Copyright © 2021 Silva, Morr, Mueller, Koch, Boehm-Sturm, Rodriguez-Sillke, Kunkel, Tzschätzsch, Kühl, Schnorr, Taupitz, Sack and Infante-Duarte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Alvarez J. I., Cayrol R., Prat A. (2011). Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta 1812 252–264. - PubMed
-
- Berndt D., Millward J. M., Schnorr J., Taupitz M., Stangl V., Paul F., et al. (2017). Inflammation-induced brain endothelial activation leads to uptake of electrostatically stabilized iron oxide nanoparticles via sulfated glycosaminoglycans. Nanomedicine 13 1411–1421. 10.1016/j.nano.2017.01.010 - DOI - PubMed
LinkOut - more resources
Full Text Sources

