Short-Chain Naphthoquinone Protects Against Both Acute and Spontaneous Chronic Murine Colitis by Alleviating Inflammatory Responses
- PMID: 34497514
- PMCID: PMC8419285
- DOI: 10.3389/fphar.2021.709973
Short-Chain Naphthoquinone Protects Against Both Acute and Spontaneous Chronic Murine Colitis by Alleviating Inflammatory Responses
Abstract
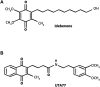
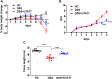
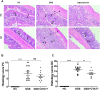
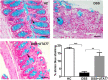
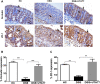
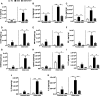
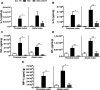
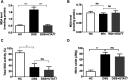
Ulcerative colitis (UC) is characterised by chronic, relapsing, idiopathic, and multifactorial colon inflammation. Recent evidence suggests that mitochondrial dysfunction plays a critical role in the onset and recurrence of this disease. Previous reports highlighted the potential of short-chain quinones (SCQs) for the treatment of mitochondrial dysfunction due to their reversible redox characteristics. We hypothesised that a recently described potent mitoprotective SCQ (UTA77) could ameliorate UC symptoms and pathology. In a dextran sodium sulphate- (DSS-) induced acute colitis model in C57BL/6J mice, UTA77 substantially improved DSS-induced body weight loss, disease activity index (DAI), colon length, and histopathology. UTA77 administration also significantly increased the expression of tight junction (TJ) proteins occludin and zona-occludin 1 (ZO-1), which preserved intestinal barrier integrity. Similar responses were observed in the spontaneous Winnie model of chronic colitis, where UTA77 significantly improved DAI, colon length, and histopathology. Furthermore, UTA77 potently suppressed elevated levels of proinflammatory cytokines and chemokines in colonic explants of both DSS-treated and Winnie mice. These results strongly suggest that UTA77 or its derivatives could be a promising novel therapeutic approach for the treatment of human UC.
Keywords: cytokines; endoplasmic reticulum stress; inflammatory bowel disease; naphthoquinones; tight junction proteins.
Copyright © 2021 Shastri, Shinde, Woolley, Smith, Gueven and Eri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses.Pharmacol Res. 2016 Aug;110:227-239. doi: 10.1016/j.phrs.2016.02.010. Epub 2016 Mar 9. Pharmacol Res. 2016. PMID: 26969793
-
Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis.World J Gastroenterol. 2017 Feb 14;23(6):999-1009. doi: 10.3748/wjg.v23.i6.999. World J Gastroenterol. 2017. PMID: 28246473 Free PMC article.
-
Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms.Int J Mol Sci. 2020 Jan 12;21(2):484. doi: 10.3390/ijms21020484. Int J Mol Sci. 2020. PMID: 31940911 Free PMC article.
-
Canna x generalis L.H. Bailey rhizome extract ameliorates dextran sulfate sodium-induced colitis via modulating intestinal mucosal dysfunction, oxidative stress, inflammation, and TLR4/ NF-ҡB and NLRP3 inflammasome pathways.J Ethnopharmacol. 2021 Apr 6;269:113670. doi: 10.1016/j.jep.2020.113670. Epub 2020 Dec 8. J Ethnopharmacol. 2021. PMID: 33301917
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota.Biomed Pharmacother. 2021 May;137:111420. doi: 10.1016/j.biopha.2021.111420. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761623
Cited by
-
Winnie Mice: A Chronic and Progressive Model of Ulcerative Colitis.Inflamm Bowel Dis. 2025 Apr 10;31(4):1158-1167. doi: 10.1093/ibd/izaf006. Inflamm Bowel Dis. 2025. PMID: 39912845 Free PMC article. Review.
-
Benefit Effect of Dendrobium officinale Ultrafine Powder on DSS-Induced Ulcerative Colitis Rats by Improving Colon Mucosal Barrier.Evid Based Complement Alternat Med. 2021 Dec 30;2021:9658638. doi: 10.1155/2021/9658638. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 35003313 Free PMC article.
References
LinkOut - more resources
Full Text Sources

