Angiotensin-Converting Enzyme 2 in the Pathogenesis of Renal Abnormalities Observed in COVID-19 Patients
- PMID: 34497535
- PMCID: PMC8419418
- DOI: 10.3389/fphys.2021.700220
Angiotensin-Converting Enzyme 2 in the Pathogenesis of Renal Abnormalities Observed in COVID-19 Patients
Abstract
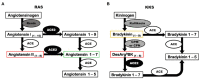
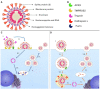
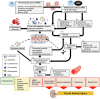
Coronavirus disease 2019 (COVID-19) was first reported in late December 2019 in Wuhan, China. The etiological agent of this disease is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the high transmissibility of the virus led to its rapid global spread and a major pandemic (ongoing at the time of writing this review). The clinical manifestations of COVID-19 can vary widely from non-evident or minor symptoms to severe acute respiratory syndrome and multi-organ damage, causing death. Acute kidney injury (AKI) has been recognized as a common complication of COVID-19 and in many cases, kidney replacement therapy (KRT) is required. The presence of kidney abnormalities on hospital admission and the development of AKI are related to a more severe presentation of COVID-19 with higher mortality rate. The high transmissibility and the broad spectrum of clinical manifestations of COVID-19 are in part due to the high affinity of SARS-CoV-2 for its receptor, angiotensin (Ang)-converting enzyme 2 (ACE2), which is widely expressed in human organs and is especially abundant in the kidneys. A debate on the role of ACE2 in the infectivity and pathogenesis of COVID-19 has emerged: Does the high expression of ACE2 promotes higher infectivity and more severe clinical manifestations or does the interaction of SARS-CoV-2 with ACE2 reduce the bioavailability of the enzyme, depleting its biological activity, which is closely related to two important physiological systems, the renin-angiotensin system (RAS) and the kallikrein-kinin system (KKS), thereby further contributing to pathogenesis. In this review, we discuss the dual role of ACE2 in the infectivity and pathogenesis of COVID-19, highlighting the effects of COVID-19-induced ACE2 depletion in the renal physiology and how it may lead to kidney injury. The ACE2 downstream regulation of KKS, that usually receives less attention, is discussed. Also, a detailed discussion on how the triad of symptoms (respiratory, inflammatory, and coagulation symptoms) of COVID-19 can indirectly promote renal injury is primary aborded.
Keywords: acute kidney injury; angiotensin-converting enzyme 2; coronavirus disease 2019; kallikrein-kinin system; renin-angiotensin system; severe acute respiratory syndrome coronavirus 2.
Copyright © 2021 Azinheira Nobrega Cruz, Gonçalves de Oliveira, Tedesco Silva Junior, Osmar Medina Pestana and Casarini.
Figures

References
-
- Aragão D. S., Cunha T. S., Arita D. Y., Andrade M. C., Fernandes A. B., Watanabe I. K., et al. (2011). Purification and characterization of angiotensin converting enzyme 2 (ACE2) from murine model of mesangial cell in culture. Int. J. Biol. Macromol. 49, 79–84. 10.1016/j.ijbiomac.2011.03.018, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

