Motilin Comparative Study: Structure, Distribution, Receptors, and Gastrointestinal Motility
- PMID: 34497583
- PMCID: PMC8419268
- DOI: 10.3389/fendo.2021.700884
Motilin Comparative Study: Structure, Distribution, Receptors, and Gastrointestinal Motility
Abstract
Motilin, produced in endocrine cells in the mucosa of the upper intestine, is an important regulator of gastrointestinal (GI) motility and mediates the phase III of interdigestive migrating motor complex (MMC) in the stomach of humans, dogs and house musk shrews through the specific motilin receptor (MLN-R). Motilin-induced MMC contributes to the maintenance of normal GI functions and transmits a hunger signal from the stomach to the brain. Motilin has been identified in various mammals, but the physiological roles of motilin in regulating GI motility in these mammals are well not understood due to inconsistencies between studies conducted on different species using a range of experimental conditions. Motilin orthologs have been identified in non-mammalian vertebrates, and the sequence of avian motilin is relatively close to that of mammals, but reptile, amphibian and fish motilins show distinctive different sequences. The MLN-R has also been identified in mammals and non-mammalian vertebrates, and can be divided into two main groups: mammal/bird/reptile/amphibian clade and fish clade. Almost 50 years have passed since discovery of motilin, here we reviewed the structure, distribution, receptor and the GI motility regulatory function of motilin in vertebrates from fish to mammals.
Keywords: comparative biology; enteric nerves; gastrointestinal contractility; motilin; motilin receptor; smooth muscle; vagus afferent nerves.
Copyright © 2021 Kitazawa and Kaiya.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
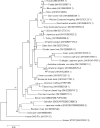

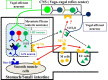
References
-
- Brown JC, Cook MA, Dryburgh JR. Motilin, A Gastric Motor Activity-Stimulating Polypeptide: Final Purification, Amino Acid Composition, and C-Terminal Residues. Gastroenterology (1972) 62:401–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

