Single-Cell Analysis of the Neonatal Immune System Across the Gestational Age Continuum
- PMID: 34497610
- PMCID: PMC8420969
- DOI: 10.3389/fimmu.2021.714090
Single-Cell Analysis of the Neonatal Immune System Across the Gestational Age Continuum
Abstract
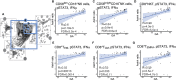
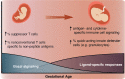
Although most causes of death and morbidity in premature infants are related to immune maladaptation, the premature immune system remains poorly understood. We provide a comprehensive single-cell depiction of the neonatal immune system at birth across the spectrum of viable gestational age (GA), ranging from 25 weeks to term. A mass cytometry immunoassay interrogated all major immune cell subsets, including signaling activity and responsiveness to stimulation. An elastic net model described the relationship between GA and immunome (R=0.85, p=8.75e-14), and unsupervised clustering highlighted previously unrecognized GA-dependent immune dynamics, including decreasing basal MAP-kinase/NFκB signaling in antigen presenting cells; increasing responsiveness of cytotoxic lymphocytes to interferon-α; and decreasing frequency of regulatory and invariant T cells, including NKT-like cells and CD8+CD161+ T cells. Knowledge gained from the analysis of the neonatal immune landscape across GA provides a mechanistic framework to understand the unique susceptibility of preterm infants to both hyper-inflammatory diseases and infections.
Keywords: Neonatal immunology; neonatal NK cells; neonatal T cells; neonatal antigen presenting cells; neonatal cytotoxic cells; prematurity.
Copyright © 2021 Peterson, Hedou, Ganio, Stelzer, Feyaerts, Harbert, Adusumelli, Ando, Tsai, Tsai, Han, Ringle, Houghteling, Reiss, Lewis, Winn, Angst, Aghaeepour, Stevenson and Gaudilliere.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

