HCV Cure With Direct-Acting Antivirals Improves Liver and Immunological Markers in HIV/HCV-Coinfected Patients
- PMID: 34497613
- PMCID: PMC8419228
- DOI: 10.3389/fimmu.2021.723196
HCV Cure With Direct-Acting Antivirals Improves Liver and Immunological Markers in HIV/HCV-Coinfected Patients
Abstract
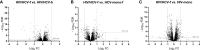
Hepatitis C virus (HCV) cure after all-oral direct-acting antiviral (DAA) therapy greatly improves the liver and immune system. We aimed to assess the impact of this HCV clearance on immune system-related markers in plasma and the gene expression profile in human immunodeficiency virus (HIV)/HCV-coinfected patients with advanced cirrhosis. We performed a prospective study on 33 HIV/HCV-coinfected patients at baseline and 36 weeks after the sustained virological response. Gene expression was evaluated by RNA-seq analysis on peripheral blood mononuclear cells (PBMCs) and plasma biomarkers by multiplex immunoassays. We found a decrease in plasma biomarkers (PD1, PDL1, CXCL10, CXCL8, IL12p70, IL10, and TGFβ) and liver disease markers (stiffness measurement (LSM), hepatic venous pressure gradient (HVPG), and transaminases, among others). Furthermore, decreased plasma levels of CXCL8, CXCL10, IL10, and PD1 were associated with reduced LSM values. We also found two upregulated (HAS1 and IRG1) and 15 downregulated (CXCL11, CCL8, CCL7, CCL2, ADARB2, RRAD, MX1, SIGLEC1, IFI44L, IFI44, IFI27, IFI6, IFIT3, IFIT1B, and IFIT1) genes at the end of follow-up, all interferon-stimulated genes (ISGs) grouped into four pathways ("cytokine-cytokine receptor interaction", "viral protein interaction with cytokine and cytokine receptor", "chemokine signaling pathway", and "hepatitis C"). Additionally, the decrease in most of these ISGs was significantly related to reduced LSM and HVPG values. In conclusion, HIV/HCV-coinfected patients with advanced-HCV-related cirrhosis who eradicated HCV following DAA therapy exhibited an improvement in liver disease markers and a significant decrease in plasma biomarkers and gene expression related to antiviral/inflammatory response, particularly in levels of several chemokines and ISGs.
Keywords: DAA therapy; HIV/HCV coinfection; PBMCs; cirrhosis; gene expression; immune system; plasma biomarkers.
Copyright © 2021 Brochado-Kith, Martínez, Berenguer, González-García, Salgüero, Sepúlveda-Crespo, Díez, Hontañón, Ibañez-Samaniego, Pérez-Latorre, Fernández-Rodríguez, Ángeles Jiménez-Sousa and Resino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Lo Re V, 3rd, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, et al. . Hepatic Decompensation in Antiretroviral-Treated Patients Co-Infected With HIV and Hepatitis C Virus Compared With Hepatitis C Virus-Monoinfected Patients: A Cohort Study. Ann Intern Med (2014) 160(6):369–79. 10.7326/M13-1829 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

