Identification and Characteristics of Fusion Peptides Derived From Enveloped Viruses
- PMID: 34497798
- PMCID: PMC8419435
- DOI: 10.3389/fchem.2021.689006
Identification and Characteristics of Fusion Peptides Derived From Enveloped Viruses
Abstract
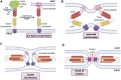
Membrane fusion events allow enveloped viruses to enter and infect cells. The study of these processes has led to the identification of a number of proteins that mediate this process. These proteins are classified according to their structure, which vary according to the viral genealogy. To date, three classes of fusion proteins have been defined, but current evidence points to the existence of additional classes. Despite their structural differences, viral fusion processes follow a common mechanism through which they exert their actions. Additional studies of the viral fusion proteins have demonstrated the key role of specific proteinogenic subsequences within these proteins, termed fusion peptides. Such peptides are able to interact and insert into membranes for which they hold interest from a pharmacological or therapeutic viewpoint. Here, the different characteristics of fusion peptides derived from viral fusion proteins are described. These criteria are useful to identify new fusion peptides. Moreover, this review describes the requirements of synthetic fusion peptides derived from fusion proteins to induce fusion by themselves. Several sequences of the viral glycoproteins E1 and E2 of HCV were, for example, identified to be able to induce fusion, which are reviewed here.
Keywords: enveloped viruses; fusion; membranotropic; peptides; secondary structures.
Copyright © 2021 Lozada, Barlow, Gonzalez, Lubin-Germain and Ballet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





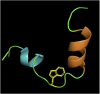

Similar articles
-
Studies of membranotropic and fusogenic activity of two putative HCV fusion peptides.Biochim Biophys Acta Biomembr. 2019 Jan;1861(1):50-61. doi: 10.1016/j.bbamem.2018.10.011. Epub 2018 Oct 19. Biochim Biophys Acta Biomembr. 2019. PMID: 30343120
-
Membranotropic peptides mediating viral entry.Pept Sci (Hoboken). 2018 Sep;110(5):e24040. doi: 10.1002/pep2.24040. Epub 2018 Feb 13. Pept Sci (Hoboken). 2018. PMID: 32328541 Free PMC article. Review.
-
Role of membranotropic sequences from herpes simplex virus type I glycoproteins B and H in the fusion process.Biochim Biophys Acta. 2010 Mar;1798(3):579-91. doi: 10.1016/j.bbamem.2010.01.006. Epub 2010 Jan 18. Biochim Biophys Acta. 2010. PMID: 20085747
-
Characterization of fusion determinants points to the involvement of three discrete regions of both E1 and E2 glycoproteins in the membrane fusion process of hepatitis C virus.J Virol. 2007 Aug;81(16):8752-65. doi: 10.1128/JVI.02642-06. Epub 2007 May 30. J Virol. 2007. PMID: 17537855 Free PMC article.
-
Membrane fusion of enveloped viruses: especially a matter of proteins.J Bioenerg Biomembr. 1990 Apr;22(2):121-55. doi: 10.1007/BF00762943. J Bioenerg Biomembr. 1990. PMID: 2109749 Review.
Cited by
-
ReaxFF-Guided Optimization of VIRIP-Based HIV-1 Entry Inhibitors.J Phys Chem B. 2025 Apr 17;129(15):3788-3795. doi: 10.1021/acs.jpcb.5c00440. Epub 2025 Apr 3. J Phys Chem B. 2025. PMID: 40178988 Free PMC article.
-
Molecular Evolution of the Fusion (F) Genes in Human Parainfluenza Virus Type 2.Microorganisms. 2025 Feb 12;13(2):399. doi: 10.3390/microorganisms13020399. Microorganisms. 2025. PMID: 40005765 Free PMC article.
-
Essential role of an ERV-derived Env38 protein in adaptive humoral immunity against an exogenous SVCV infection in a zebrafish model.PLoS Pathog. 2023 Apr 4;19(4):e1011222. doi: 10.1371/journal.ppat.1011222. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37014912 Free PMC article.
-
Machine and deep learning to predict viral fusion peptides.Comput Struct Biotechnol J. 2025 Feb 18;27:692-704. doi: 10.1016/j.csbj.2025.02.011. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40083606 Free PMC article.
-
Centrifugation-Based Purification Protocol Optimization Enhances Structural Preservation of Nucleopolyhedrovirus Budded Virion Envelopes.Insects. 2025 Apr 17;16(4):424. doi: 10.3390/insects16040424. Insects. 2025. PMID: 40332984 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

