Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and Computational Study of a Novel Organic Salt Obtained from Benzylamine and an Acidic Component
- PMID: 34497924
- PMCID: PMC8412916
- DOI: 10.1021/acsomega.1c03078
Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and Computational Study of a Novel Organic Salt Obtained from Benzylamine and an Acidic Component
Abstract
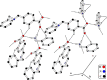
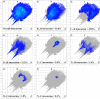
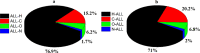
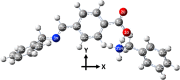
A novel Schiff base compound named as phenylmethanaminium (E)-4-((benzylimino)methyl)benzoate C7H10N+. C15H12NO2 - (A) is synthesized by the chemical reaction of benzylamine and 4-carboxybenzaldehyde in ethanol, and the structure of the titled compound is verified using the single-crystal X-ray diffraction technique. Structural investigation inferred that the crystal packing is mainly stabilized by N-H···O and comparatively weak C-H···O bonding between the cation and anion and further stabilized by weak C-H···π and C-O···π interactions. Hirshfeld surface analysis is employed to explore the noncovalent interactions that are responsible for crystal packing quantitatively. Furthermore, we have used state-of-the-art quantum chemical calculations to get comprehensive insights into the structure-optoelectronic property relationship for the entitled compound. The molecular geometry of compound A is optimized at the M06/6-311G* level of theory. The linear polarizability, third-order nonlinear optical (NLO) polarizability, total and partial density of states, and UV-visible spectrum are calculated through quantum chemical calculations. We believe that compound A is not only a new addition to crystallographic data but also possesses good optical and NLO properties for its potential use in lasers and frequency-converting applications.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Single-Crystal Investigation, Hirshfeld Surface Analysis, and DFT Study of Third-Order NLO Properties of Unsymmetrical Acyl Thiourea Derivatives.ACS Omega. 2021 Nov 12;6(46):31211-31225. doi: 10.1021/acsomega.1c04884. eCollection 2021 Nov 23. ACS Omega. 2021. PMID: 34841164 Free PMC article.
-
Synthesis, crystal structure, Hirshfeld surface analysis and experimental and theoretical study of new azo compound methyl 2-{2-[(E)-2-oxo-1,2-dihydronaphthalen-1-ylidene]hydrazin-1-yl}benzoate.Acta Crystallogr C Struct Chem. 2025 Jun 1;81(Pt 6):319-337. doi: 10.1107/S2053229625003523. Epub 2025 May 13. Acta Crystallogr C Struct Chem. 2025. PMID: 40358120
-
Synthesis of Crystalline Fluoro-Functionalized Imines, Single Crystal Investigation, Hirshfeld Surface Analysis, and Theoretical Exploration.ACS Omega. 2022 Mar 14;7(11):9867-9878. doi: 10.1021/acsomega.2c00288. eCollection 2022 Mar 22. ACS Omega. 2022. PMID: 35356686 Free PMC article.
-
Synthesis, crystal structure determination and Hirshfeld surface analysis of three new salt forms of creatinine with hydrobromic acid, 3-aminobenzoic acid and 3,5-dinitrobenzoic acid.Acta Crystallogr C Struct Chem. 2022 Aug 1;78(Pt 8):437-448. doi: 10.1107/S2053229622006684. Epub 2022 Jul 11. Acta Crystallogr C Struct Chem. 2022. PMID: 35924362
-
Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino] prop-2-enoic acid.Spectrochim Acta A Mol Biomol Spectrosc. 2016 Jan 15;153:625-36. doi: 10.1016/j.saa.2015.09.002. Epub 2015 Sep 6. Spectrochim Acta A Mol Biomol Spectrosc. 2016. PMID: 26452098
Cited by
-
Efficient Synthesis of Imine-Carboxylic Acid Functionalized Compounds: Single Crystal, Hirshfeld Surface and Quantum Chemical Exploration.Molecules. 2023 Mar 27;28(7):2967. doi: 10.3390/molecules28072967. Molecules. 2023. PMID: 37049730 Free PMC article.
-
Shedding Light on the Synthesis, Crystal Structure, Characterization, and Computational Study of Optoelectronic Properties and Bioactivity of Imine derivatives.ACS Omega. 2022 Feb 2;7(6):5217-5230. doi: 10.1021/acsomega.1c06325. eCollection 2022 Feb 15. ACS Omega. 2022. PMID: 35187337 Free PMC article.
-
Synthesis, Structural, and Intriguing Electronic Properties of Symmetrical Bis-Aryl-α,β-Unsaturated Ketone Derivatives.ACS Omega. 2022 Oct 20;7(43):39294-39309. doi: 10.1021/acsomega.2c05441. eCollection 2022 Nov 1. ACS Omega. 2022. PMID: 36340158 Free PMC article.
-
Rational synthesis, biological screening of azo derivatives of chloro-phenylcarbonyl diazenyl hydroxy dipyrimidines/thioxotetrahydropyrimidines and their metal complexes.Heliyon. 2022 Dec 27;9(1):e12492. doi: 10.1016/j.heliyon.2022.e12492. eCollection 2023 Jan. Heliyon. 2022. PMID: 36699273 Free PMC article.
-
A hybrid molecularly imprinted ratiometric fluorescent probe based on Eu-MOFs cross-linked fluorescence enhancement for smartphone-assisted detection of dicamba.Mikrochim Acta. 2025 Feb 22;192(3):181. doi: 10.1007/s00604-025-07028-w. Mikrochim Acta. 2025. PMID: 39987327
References
-
- Munawar K. S.; Haroon S. M.; Haroon S. M.; Hussain S. A.; Raza H. Schiff bases: multipurpose pharmacophores with extensive biological applications. J. Basic Appl. Sci. 2018, 14, 217–229. 10.6000/1927-5129.2018.14.34. - DOI
-
- Malakyan M.; Babayan N.; Grigoryan R.; Sarkisyan N.; Tonoyan V.; Tadevosyan D.; Matosyan V.; Aroutiounian R.; Arakelyan A. Synthesis, characterization and toxicity studies of pyridinecarboxaldehydes and L-tryptophan derived Schiff bases and corresponding copper (II) complexes. F1000Research 2016, 5, 1921–1932. 10.12688/f1000research.9226.1. - DOI - PMC - PubMed
-
- Rauf A.; Shah A.; Munawar K. S.; Ali S.; Nawaz Tahir M.; Javed M.; Khan A. M. Synthesis, physicochemical elucidation, biological screening and molecular docking studies of a Schiff base and its metal(II) complexes. Arabian J. Chem. 2020, 13, 1130–1141. 10.1016/j.arabjc.2017.09.015. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

