Meningococcal Detoxified Outer Membrane Vesicle Vaccines Enhance Gonococcal Clearance in a Murine Infection Model
- PMID: 34498079
- PMCID: PMC8844591
- DOI: 10.1093/infdis/jiab450
Meningococcal Detoxified Outer Membrane Vesicle Vaccines Enhance Gonococcal Clearance in a Murine Infection Model
Abstract
Background: Despite decades of research efforts, development of a gonorrhea vaccine has remained elusive. Epidemiological studies suggest that detoxified outer membrane vesicle (dOMV) vaccines from Neisseria meningitidis (Nm) may protect against infection with Neisseria gonorrhoeae (Ng). We recently reported that Nm dOMVs lacking the major outer membrane proteins (OMPs) PorA, PorB, and RmpM induced greater antibody cross-reactivity against heterologous Nm strains than wild-type (WT) dOMVs and may represent an improved vaccine against gonorrhea.
Methods: We prepared dOMV vaccines from meningococcal strains that were sufficient or deleted for PorA, PorB, and RmpM. Vaccines were tested in a murine genital tract infection model and antisera were used to identify vaccine targets.
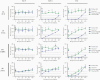
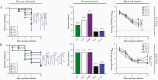
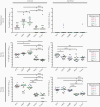
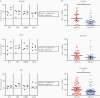
Results: Immunization with Nm dOMVs significantly and reproducibly enhanced gonococcal clearance for mice immunized with OMP-deficient dOMVs; significant clearance for WT dOMV-immunized mice was observed in one of two experiments. Clearance was associated with serum and vaginal anti-Nm dOMV immunoglobulin G (IgG) antibodies that cross-reacted with Ng. Serum IgG was used to identify putative Ng vaccine targets, including PilQ, MtrE, NlpD, and GuaB.
Conclusions: Meningococcal dOMVs elicited a protective effect against experimental gonococcal infection. Recognition and identification of Ng vaccine targets by Nm dOMV-induced antibodies supports the development of a cross-protective Neisseria vaccine.
Keywords: Neisseria gonorrhoeae; Neisseria meningitidis; OMV; gonococcus; meningococcus; vaccine.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures

Comment in
-
Outer membrane vesicle vaccines for Neisseria gonorrhoeae.Nat Rev Urol. 2022 Jan;19(1):5-6. doi: 10.1038/s41585-021-00534-5. Nat Rev Urol. 2022. PMID: 34716431 Free PMC article.
References
-
- World Health Organization. Report on global sexually transmitted infection surveillance, 2018. Geneva, Switzerland: WHO, 2018.
-
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. Atlanta, Georgia: CDC, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

