Cell Transplantation to Restore Lost Auditory Nerve Function is a Realistic Clinical Opportunity
- PMID: 34498511
- PMCID: PMC8438274
- DOI: 10.1177/09636897211035076
Cell Transplantation to Restore Lost Auditory Nerve Function is a Realistic Clinical Opportunity
Abstract
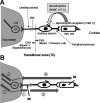
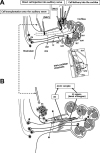
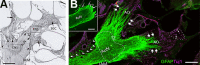
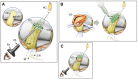
Hearing is one of our most important means of communication. Disabling hearing loss (DHL) is a long-standing, unmet problem in medicine, and in many elderly people, it leads to social isolation, depression, and even dementia. Traditionally, major efforts to cure DHL have focused on hair cells (HCs). However, the auditory nerve is also important because it transmits electrical signals generated by HCs to the brainstem. Its function is critical for the success of cochlear implants as well as for future therapies for HC regeneration. Over the past two decades, cell transplantation has emerged as a promising therapeutic option for restoring lost auditory nerve function, and two independent studies on animal models show that cell transplantation can lead to functional recovery. In this article, we consider the approaches most likely to achieve success in the clinic. We conclude that the structure and biochemical integrity of the auditory nerve is critical and that it is important to preserve the remaining neural scaffold, and in particular the glial scar, for the functional integration of donor cells. To exploit the natural, autologous cell scaffold and to minimize the deleterious effects of surgery, donor cells can be placed relatively easily on the surface of the nerve endoscopically. In this context, the selection of donor cells is a critical issue. Nevertheless, there is now a very realistic possibility for clinical application of cell transplantation for several different types of hearing loss.
Keywords: auditory nerve; cell transplantation; glial scar; nerve regeneration; scaffold.
Conflict of interest statement
Figures

Similar articles
-
"Replacement of diseased auditory neurons by cell transplantation".Front Biosci. 2008 Jan 1;13:2165-76. doi: 10.2741/2832. Front Biosci. 2008. PMID: 17981700 Review.
-
Cells transplanted onto the surface of the glial scar reveal hidden potential for functional neural regeneration.Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):E3431-40. doi: 10.1073/pnas.1501835112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080415 Free PMC article.
-
Rebuilding lost hearing using cell transplantation.Neurosurgery. 2007 Mar;60(3):417-33; discussion 433. doi: 10.1227/01.NEU.0000249189.46033.42. Neurosurgery. 2007. PMID: 17327786 Review.
-
Mutation of OPA1 gene causes deafness by affecting function of auditory nerve terminals.Brain Res. 2009 Dec 1;1300:97-104. doi: 10.1016/j.brainres.2009.08.083. Epub 2009 Sep 3. Brain Res. 2009. PMID: 19733158
-
The Therapeutic Dilemma of Cochlear Nerve Deficiency: Cochlear or Brainstem Implantation?Otolaryngol Head Neck Surg. 2014 Aug;151(2):308-14. doi: 10.1177/0194599814531913. Epub 2014 Apr 23. Otolaryngol Head Neck Surg. 2014. PMID: 24759909
Cited by
-
Characteristics and needs of physical and mental health among older adult individuals with disabilities under the background of smart healthcare: according to data from the China family panel studies.Front Public Health. 2025 Feb 21;13:1475466. doi: 10.3389/fpubh.2025.1475466. eCollection 2025. Front Public Health. 2025. PMID: 40061462 Free PMC article.
-
Age-Related Hearing Loss: Sensory and Neural Etiology and Their Interdependence.Front Aging Neurosci. 2022 Feb 17;14:814528. doi: 10.3389/fnagi.2022.814528. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250542 Free PMC article. Review.
-
Potential uses of auditory nerve stimulation to modulate immune responses in the inner ear and auditory brainstem.Front Integr Neurosci. 2023 Dec 14;17:1294525. doi: 10.3389/fnint.2023.1294525. eCollection 2023. Front Integr Neurosci. 2023. PMID: 38162822 Free PMC article.
-
Is there an unmet medical need for improved hearing restoration?EMBO Mol Med. 2022 Aug 8;14(8):e15798. doi: 10.15252/emmm.202215798. Epub 2022 Jul 14. EMBO Mol Med. 2022. PMID: 35833443 Free PMC article. Review.
-
Photobiomodulation Can Enhance Stem Cell Viability in Cochlea with Auditory Neuropathy but Does Not Restore Hearing.Stem Cells Int. 2023 Nov 15;2023:6845571. doi: 10.1155/2023/6845571. eCollection 2023. Stem Cells Int. 2023. PMID: 38020205 Free PMC article.
References
-
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, et al.Dementia prevention, intervention, and care. Lancet 2017;390(10113):2673–2734. - PubMed
-
- Sekiya T, Kojima K, Matsumoto M, Holley MC, Ito J. Rebuilding lost hearing using cell transplantation. Neurosurgery. 2007;60(3):417–433; discussion 433. - PubMed
-
- Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol. 2001;6(6):305–318. - PubMed
-
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 2002;25(1):51–101. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

