Perioperative Pulmonary Atelectasis: Part I. Biology and Mechanisms
- PMID: 34499087
- PMCID: PMC9869183
- DOI: 10.1097/ALN.0000000000003943
Perioperative Pulmonary Atelectasis: Part I. Biology and Mechanisms
Abstract
Pulmonary atelectasis is common in the perioperative period. Physiologically, it is produced when collapsing forces derived from positive pleural pressure and surface tension overcome expanding forces from alveolar pressure and parenchymal tethering. Atelectasis impairs blood oxygenation and reduces lung compliance. It is increasingly recognized that it can also induce local tissue biologic responses, such as inflammation, local immune dysfunction, and damage of the alveolar-capillary barrier, with potential loss of lung fluid clearance, increased lung protein permeability, and susceptibility to infection, factors that can initiate or exaggerate lung injury. Mechanical ventilation of a heterogeneously aerated lung (e.g., in the presence of atelectatic lung tissue) involves biomechanical processes that may precipitate further lung damage: concentration of mechanical forces, propagation of gas-liquid interfaces, and remote overdistension. Knowledge of such pathophysiologic mechanisms of atelectasis and their consequences in the healthy and diseased lung should guide optimal clinical management.
Copyright © 2021, the American Society of Anesthesiologists. All Rights Reserved.
Conflict of interest statement
Figures


 ) is responsible for loss of expansion and reduced alveolar ventilation (⩒A). Increased alveolar gas absorption (
) is responsible for loss of expansion and reduced alveolar ventilation (⩒A). Increased alveolar gas absorption ( ) reduces intraluminal alveolar pressure (Palv). Low ⩒A/Q̇, high FIO2 and low mixed venous oxygen partial pressure (P O2) may participate in such gas exchange imbalance. Quantitative or qualitative surfactant impairment leads to higher surface tension and facilitates alveolar collapse (
) reduces intraluminal alveolar pressure (Palv). Low ⩒A/Q̇, high FIO2 and low mixed venous oxygen partial pressure (P O2) may participate in such gas exchange imbalance. Quantitative or qualitative surfactant impairment leads to higher surface tension and facilitates alveolar collapse ( ). Pc’O2 = end-capillary oxygen partial pressure.
). Pc’O2 = end-capillary oxygen partial pressure.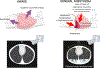

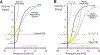
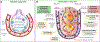

Comment in
-
Perioperative Pulmonary Atelectasis: Comment.Anesthesiology. 2022 Jul 1;137(1):125-126. doi: 10.1097/ALN.0000000000004231. Anesthesiology. 2022. PMID: 35486841 No abstract available.
References
-
- Bendixen HH, Hedley-Whyte J, Laver MB: Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med 1963; 269:991–6 - PubMed
-
- Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L: Pulmonary densities during anesthesia with muscular relaxation--a proposal of atelectasis. Anesthesiology 1985; 62:422–8 - PubMed
-
- Futier E, Constantin J-M, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant J-Y, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin J-E, Pereira B, Jaber S: A Trial of Intraoperative Low-Tidal-Volume Ventilation in Abdominal Surgery. N Engl J Med 2013; 369:428–37 - PubMed
-
- Mead J, Takishima T, Leith D: Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970; 28:596–608 - PubMed
-
- Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O: The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 2017; 50 - PubMed

