Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis
- PMID: 34499128
- PMCID: PMC9088512
- DOI: 10.1093/brain/awab311
Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis
Abstract
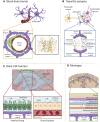
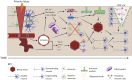
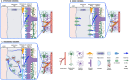
Aquaporin channels facilitate bidirectional water flow in all cells and tissues. AQP4 is highly expressed in astrocytes. In the CNS, it is enriched in astrocyte endfeet, at synapses, and at the glia limitans, where it mediates water exchange across the blood-spinal cord and blood-brain barriers (BSCB/BBB), and controls cell volume, extracellular space volume, and astrocyte migration. Perivascular enrichment of AQP4 at the BSCB/BBB suggests a role in glymphatic function. Recently, we have demonstrated that AQP4 localization is also dynamically regulated at the subcellular level, affecting membrane water permeability. Ageing, cerebrovascular disease, traumatic CNS injury, and sleep disruption are established and emerging risk factors in developing neurodegeneration, and in animal models of each, impairment of glymphatic function is associated with changes in perivascular AQP4 localization. CNS oedema is caused by passive water influx through AQP4 in response to osmotic imbalances. We have demonstrated that reducing dynamic relocalization of AQP4 to the BSCB/BBB reduces CNS oedema and accelerates functional recovery in rodent models. Given the difficulties in developing pore-blocking AQP4 inhibitors, targeting AQP4 subcellular localization opens up new treatment avenues for CNS oedema, neurovascular and neurodegenerative diseases, and provides a framework to address fundamental questions about water homeostasis in health and disease.
Keywords: neurodegeneration; regulation; traumatic brain and spinal cord injury; water channel.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Manley GT, Binder DK, Papadopoulos MC, Verkman AS.. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129(4):983–991. - PubMed
-
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC.. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758(8):1085–1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/N029453/1/MRC_/Medical Research Council/United Kingdom
- G-1003/PUK_/Parkinson's UK/United Kingdom
- MR/P007058/1/MRC_/Medical Research Council/United Kingdom
- H-1102/PUK_/Parkinson's UK/United Kingdom
- H-1301/PUK_/Parkinson's UK/United Kingdom
- MC_EX_MR/N50192X/1/MRC_/Medical Research Council/United Kingdom
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MR/M024962/1/MRC_/Medical Research Council/United Kingdom
- PG/10/54/28460/BHF_/British Heart Foundation/United Kingdom
- K-1003/PUK_/Parkinson's UK/United Kingdom
- J-0901/PUK_/Parkinson's UK/United Kingdom
- R01 NS089709/NS/NINDS NIH HHS/United States
- G-0801/PUK_/Parkinson's UK/United Kingdom
- BB/D012910/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom

