Mild and Chemoselective Phosphorylation of Alcohols Using a Ψ-Reagent
- PMID: 34499517
- PMCID: PMC8733960
- DOI: 10.1021/acs.orglett.1c02736
Mild and Chemoselective Phosphorylation of Alcohols Using a Ψ-Reagent
Abstract
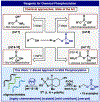
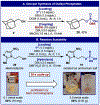
An operationally simple, scalable, and chemoselective method for the direct phosphorylation of alcohols using a P(V)-approach based on the Ψ-reagent platform is disclosed. The method features a broad substrate scope of utility in both simple and complex settings and provides access to valuable phosphorylated alcohols that would be otherwise difficult to obtain.
Figures


References
-
- Hunter T Why nature chose phosphate to modify proteins. Phil. Trans. R. Soc. B 2012, 367, 2513–2516. - PMC - PubMed
- Johnson LN; Lewis RJ Structural Basis for Control by Phosphorylation. Chem Rev. 2001, 101, 2209–2242. - PubMed
- Berridge MJ; Irvine RF Inositol phosphates and cell signalling. Nature 1989, 341, 197–205. - PubMed
-
- Phosphorus. An Outline of its Chemistry, Biochemistry and Technology 4th Ed.; Corbridge D. E. C., Ed.; Elsevier, 1990, pp. 495–554.
-
- Bhat GA; Kalita A. Ch.; Murugavel R Intriguing structural chemistry of neutral and anionic layered monoalkylphosphates: single-source precursors for high-yield ceramic phosphates. CrystEngComm 2017, 19, 5390–5401.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

