Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients
- PMID: 34499614
- PMCID: PMC8457592
- DOI: 10.18632/aging.203485
Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients
Abstract
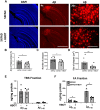
Vascular dysfunction is entwined with aging and in the pathogenesis of Alzheimer's disease (AD) and contributes to reduced cerebral blood flow (CBF) and consequently, hypoxia. Hyperbaric oxygen therapy (HBOT) is in clinical use for a wide range of medical conditions. In the current study, we exposed 5XFAD mice, a well-studied AD model that presents impaired cognitive abilities, to HBOT and then investigated the therapeutical effects using two-photon live animal imaging, behavioral tasks, and biochemical and histological analysis. HBOT increased arteriolar luminal diameter and elevated CBF, thus contributing to reduced hypoxia. Furthermore, HBOT reduced amyloid burden by reducing the volume of pre-existing plaques and attenuating the formation of new ones. This was associated with changes in amyloid precursor protein processing, elevated degradation and clearance of Aß protein and improved behavior of 5XFAD mice. Hence, our findings are consistent with the effects of HBOT being mediated partially through a persistent structural change in blood vessels that reduces brain hypoxia. Motivated by these findings, we exposed elderly patients with significant memory loss at baseline to HBOT and observed an increase in CBF and improvement in cognitive performances. This study demonstrates HBOT efficacy in hypoxia-related neurological conditions, particularly in AD and aging.
Keywords: Alzheimer's disease; amyloid burden; cerebral blood flow; hyperbaric oxygen therapy; vascular dysfunction.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

