Hospitalizations Associated with COVID-19 Among Children and Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021
- PMID: 34499627
- PMCID: PMC8437052
- DOI: 10.15585/mmwr.mm7036e2
Hospitalizations Associated with COVID-19 Among Children and Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021
Abstract
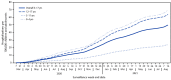
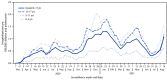
Although COVID-19-associated hospitalizations and deaths have occurred more frequently in adults,† COVID-19 can also lead to severe outcomes in children and adolescents (1,2). Schools are opening for in-person learning, and many prekindergarten children are returning to early care and education programs during a time when the number of COVID-19 cases caused by the highly transmissible B.1.617.2 (Delta) variant of SARS-CoV-2, the virus that causes COVID-19, is increasing.§ Therefore, it is important to monitor indicators of severe COVID-19 among children and adolescents. This analysis uses Coronavirus Disease 2019-Associated Hospitalization Surveillance Network (COVID-NET)¶ data to describe COVID-19-associated hospitalizations among U.S. children and adolescents aged 0-17 years. During March 1, 2020-August 14, 2021, the cumulative incidence of COVID-19-associated hospitalizations was 49.7 per 100,000 children and adolescents. The weekly COVID-19-associated hospitalization rate per 100,000 children and adolescents during the week ending August 14, 2021 (1.4) was nearly five times the rate during the week ending June 26, 2021 (0.3); among children aged 0-4 years, the weekly hospitalization rate during the week ending August 14, 2021, was nearly 10 times that during the week ending June 26, 2021.** During June 20-July 31, 2021, the hospitalization rate among unvaccinated adolescents (aged 12-17 years) was 10.1 times higher than that among fully vaccinated adolescents. Among all hospitalized children and adolescents with COVID-19, the proportions with indicators of severe disease (such as intensive care unit [ICU] admission) after the Delta variant became predominant (June 20-July 31, 2021) were similar to those earlier in the pandemic (March 1, 2020-June 19, 2021). Implementation of preventive measures to reduce transmission and severe outcomes in children is critical, including vaccination of eligible persons, universal mask wearing in schools, recommended mask wearing by persons aged ≥2 years in other indoor public spaces and child care centers,†† and quarantining as recommended after exposure to persons with COVID-19.§§.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. William Schaffner reports consultant fees from VBI Vaccines, outside the submitted work. Eli Shiltz and Laurie M. Billing report grant funding from the Council of State and Territorial Epidemiologists (CSTE) for the population-based Influenza Hospitalization Surveillance Project (IHSP) and COVID-NET activities. Lauren Leegwater and Sue Kim report grant support from CSTE through the Michigan Department of Health and Human Services. Andy Weigel and Kenzie Teno report grant support from CSTE for data collection and participation in ongoing meetings related to COVID-19 hospitalization surveillance. Evan J. Anderson reports grants for clinical trials from Pfizer, Merck, PaxVax, Micron, Sanofi-Pasteur, Janssen, MedImmune, and GSK; consulting fees from Sanofi-Pasteur, Pfizer, Janssen, and Medscape; personal fees for data safety monitoring board participation from Kentucky Bioprocessing, Inc. and Sanofi-Pasteur; and institutional funding from the National Institutes of Health to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. No other potential conflicts of interest were disclosed.
Figures
References
-
- Kim L, Whitaker M, O’Halloran A, et al.; COVID-NET Surveillance Team. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081–8. 10.15585/mmwr.mm6932e3 - DOI - PMC - PubMed
-
- Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12–15 years—United States, May 2021. MMWR Morb Mortal Wkly Rep 2021;70:749–52. 10.15585/mmwr.mm7020e1 - DOI - PMC - PubMed
-
- Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163–6. 10.15585/mmwr.mm7034e3 - DOI - PMC - PubMed

