Notes from the Field: Tuberculosis Outbreak Linked to a Contaminated Bone Graft Product Used in Spinal Surgery - Delaware, March-June 2021
- PMID: 34499629
- PMCID: PMC8437057
- DOI: 10.15585/mmwr.mm7036a4
Notes from the Field: Tuberculosis Outbreak Linked to a Contaminated Bone Graft Product Used in Spinal Surgery - Delaware, March-June 2021
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
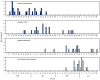
Figures
References
-
- Food and Drug Administration. Urgent voluntary notification: FiberCel Fiber Viable Bone Matrix (“FiberCel”)—lot number: NMDS210011. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/vaccines-blood-biologics/recalls-biologics/urgent-vo...
-
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/infectioncontrol/pdf/guidelines/isolation-guidelines... - PubMed
-
- National Tuberculosis Controllers Association. CDC. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005;54(No. RR-15). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

