Systemic adverse events after screening of retinopathy of prematurity with mydriatic
- PMID: 34499693
- PMCID: PMC8428556
- DOI: 10.1371/journal.pone.0256878
Systemic adverse events after screening of retinopathy of prematurity with mydriatic
Abstract
Purpose: To evaluate systemic adverse events after screening for retinopathy of prematurity (ROP) performed with mydriatic.
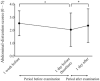
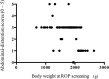
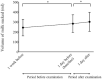
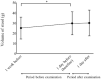
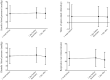
Methods: This was a retrospective case series study. Medical records of consecutive patients who underwent screening for ROP with 0.5% phenylephrine and 0.5% tropicamide eyedrops were retrospectively reviewed. The score of abdominal distention (0-5), volume of milk sucked and volume of stool, along with systemic details (pulse and respiration rates, blood pressure and number of periods of apnea) were collected at 1 week and 1 day before ROP examination, and at 1 day after examination. Results were compared between the days before and after examination. Correlation between body weight at the time of examination and the score of abdominal distention was examined. The numbers of infants with abdominal and/or systemic adverse events were compared between pre- and post-examination periods.
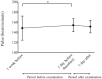
Results: Eighty-six infants met the inclusion criteria. The score of abdominal distention increased from 2.0 at 1 day before examination to 2.3 at 1 day after examination (p = 0.005), and the number of infants who had worsened abdominal distension increased after examination (p = 0.01). Infants with lower body weight had a higher score of abdominal distention (p < 0.0001, r = -0.57). The number of infants with reduced milk consumption increased after examination (p = 0.0001), as did the number of infants with decreased pulse rate (p = 0.0008).
Conclusions: Screening for ROP with mydriatic may have adverse effects on systemic conditions. Infants should be carefully monitored after ROP screening with mydriatic.
Conflict of interest statement
M.O. reports personal fees from Santen Pharmaceutical, unrelated to the submitted work. We used Midorin-PTM eye drop marketed by Santen Pharmaceutical, a mixture of 0.5% phenylephrine and 0.5% tropicamide, as mydriatics which is commonly used in Japan. The other authors declare that they have no conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Rengstorff RH, Doughty CB. Mydriatic and cycloplegic drugs: a review of ocular and systemic complications. Am J Optom Physiol Opt. 1982;59(2):162–77. Epub 1982/02/01. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

