Biofilm regulation in Clostridioides difficile: Novel systems linked to hypervirulence
- PMID: 34499698
- PMCID: PMC8428550
- DOI: 10.1371/journal.ppat.1009817
Biofilm regulation in Clostridioides difficile: Novel systems linked to hypervirulence
Abstract
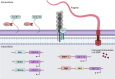
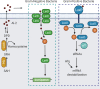
Clostridiodes difficile (C. difficile) was ranked an "urgent threat" by the Centers for Disease Control and Prevention (CDC) in 2019. C. difficile infection (CDI) is the most common healthcare-associated infection (HAI) in the United States of America as well as the leading cause of antibiotic-associated gastrointestinal disease. C. difficile is a gram-positive, rod-shaped, spore-forming, anaerobic bacterium that causes infection of the epithelial lining of the gut. CDI occurs most commonly after disruption of the human gut microflora following the prolonged use of broad-spectrum antibiotics. However, the recurrent nature of this disease has led to the hypothesis that biofilm formation may play a role in its pathogenesis. Biofilms are sessile communities of bacteria protected from extracellular stresses by a matrix of self-produced proteins, polysaccharides, and extracellular DNA. Biofilm regulation in C. difficile is still incompletely understood, and its role in disease recurrence has yet to be fully elucidated. However, many factors have been found to influence biofilm formation in C. difficile, including motility, adhesion, and hydrophobicity of the bacterial cells. Small changes in one of these systems can greatly influence biofilm formation. Therefore, the biofilm regulatory system would need to coordinate all these systems to create optimal biofilm-forming physiology under appropriate environmental conditions. The coordination of these systems is complex and multifactorial, and any analysis must take into consideration the influences of the stress response, quorum sensing (QS), and gene regulation by second messenger molecule cyclic diguanosine monophosphate (c-di-GMP). However, the differences in biofilm-forming ability between C. difficile strains such as 630 and the "hypervirulent" strain, R20291, make it difficult to assign a "one size fits all" mechanism to biofilm regulation in C. difficile. This review seeks to consolidate published data regarding the regulation of C. difficile biofilms in order to identify gaps in knowledge and propose directions for future study.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, et al.. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet. 2006;38:779–786. Available from: https://www.nature.com/articles/ng1830. doi: 10.1038/ng1830 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

