Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine
- PMID: 34500691
- PMCID: PMC8433626
- DOI: 10.3390/molecules26175258
Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine
Abstract
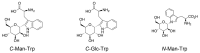
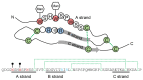
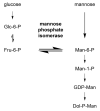
C-Mannosylation is a post-translational modification of proteins in the endoplasmic reticulum. Monomeric α-mannose is attached to specific Trp residues at the first Trp in the Trp-x-x-Trp/Cys (W-x-x-W/C) motif of substrate proteins, by the action of C-mannosyltransferases, DPY19-related gene products. The acceptor substrate proteins are included in the thrombospondin type I repeat (TSR) superfamily, cytokine receptor type I family, and others. Previous studies demonstrated that C-mannosylation plays critical roles in the folding, sorting, and/or secretion of substrate proteins. A C-mannosylation-defective gene mutation was identified in humans as the disease-associated variant affecting a C-mannosylation motif of W-x-x-W of ADAMTSL1, which suggests the involvement of defects in protein C-mannosylation in human diseases such as developmental glaucoma, myopia, and/or retinal defects. On the other hand, monomeric C-mannosyl Trp (C-Man-Trp), a deduced degradation product of C-mannosylated proteins, occurs in cells and extracellular fluids. Several studies showed that the level of C-Man-Trp is upregulated in blood of patients with renal dysfunction, suggesting that the metabolism of C-Man-Trp may be involved in human kidney diseases. Together, protein C-mannosylation is considered to play important roles in the biosynthesis and functions of substrate proteins, and the altered regulation of protein C-manosylation may be involved in the pathophysiology of human diseases. In this review, we consider the biochemical and biomedical knowledge of protein C-mannosylation and C-Man-Trp, and introduce recent studies concerning their significance in biology and medicine.
Keywords: C-mannosyl tryptophan; C-mannosylation; DPY19; cytokine receptor type I; thrombospondin type I repeat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ihara Y., Inai Y., Ikezaki M., Matsui I.L., Manabe S., Ito Y. C-Mannosylation: Modification on tryptophan in cellular proteins. In: Taniguchi N., Endo T., Hart G.W., Seeberger P.H., Wong C.H., editors. Glycoscience: Biology and Medicine. Volume 2. Springer; Tokyo, Japan: 2015. pp. 1091–1099.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

