An Evaluation of Understudied Phytocannabinoids and Their Effects in Two Neuronal Models
- PMID: 34500785
- PMCID: PMC8434068
- DOI: 10.3390/molecules26175352
An Evaluation of Understudied Phytocannabinoids and Their Effects in Two Neuronal Models
Abstract
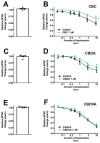
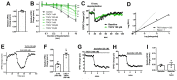
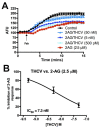
Cannabis contains more than 100 phytocannabinoids. Most of these remain poorly characterized, particularly in neurons. We tested a panel of five phytocannabinoids-cannabichromene (CBC), cannabidiolic acid (CBDA), cannabidivarin (CBDV), cannabidivarinic acid (CBDVA), and Δ9-tetrahydrocannabivarin (THCV) in two neuronal models, autaptic hippocampal neurons and dorsal root ganglion (DRG) neurons. Autaptic neurons expressed a form of CB1-dependent retrograde plasticity while DRGs expressed a variety of transient receptor potential (TRP) channels. CBC, CBDA, and CBDVA had little or no effect on neuronal cannabinoid signaling. CBDV and THCV differentially inhibited cannabinoid signaling. THCV inhibited CB1 receptors presynaptically while CBDV acted post-synaptically, perhaps by inhibiting 2-AG production. None of the compounds elicited a consistent DRG response. In summary, we find that two of five 'minor' phytocannabinoids tested antagonized CB1-based signaling in a neuronal model, but with very different mechanisms. Our findings highlight the diversity of potential actions of phytocannabinoids and the importance of fully evaluating these compounds in neuronal models.
Keywords: cannabichromene; cannabidiolic acid; cannabidivarin; cannabidivarinic acid; phytocannabinoids; tetrahydrocannabivarin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gaoni Y., Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

