Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air
- PMID: 34500823
- PMCID: PMC8433652
- DOI: 10.3390/molecules26175390
Porphyrin N-Pincer Pd(II)-Complexes in Water: A Base-Free and Nature-Inspired Protocol for the Oxidative Self-Coupling of Potassium Aryltrifluoroborates in Open-Air
Abstract
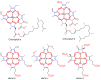
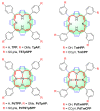
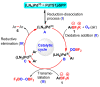
Metalloporphyrins (and porphyrins) are well known as pigments of life in nature, since representatives of this group include chlorophylls (Mg-porphyrins) and heme (Fe-porphyrins). Hence, the construction of chemistry based on these substances can be based on the imitation of biological systems. Inspired by nature, in this article we present the preparation of five different porphyrin, meso-tetraphenylporphyrin (TPP), meso-tetra(p-anisyl)porphyrin (TpAP), tetrasodium meso-tetra(p-sulfonatophenyl)porphyrin (TSTpSPP), meso-tetra(m-hydroxyphenyl)porphyrin (TmHPP), and meso-tetra(m-carboxyphenyl)porphyrin (TmCPP) as well as their N-pincer Pd(II)-complexes such as Pd(II)-meso-tetraphenylporphyrin (PdTPP), Pd(II)-meso-tetra(p-anisyl)porphyrin (PdTpAP), Pd(II)-tetrasodium meso-tetra(p-sulfonatophenyl)porphyrin (PdTSTpSPP), Pd(II)-meso-tetra(m-hydroxyphenyl)porphyrin (PdTmHPP), and Pd(II)-meso-tetra(m-carboxyphenyl)porphyrin (PdTmCPP). These porphyrin N-pincer Pd(II)-complexes were studied and found to be effective in the base-free self-coupling reactions of potassium aryltrifluoroborates (PATFBs) in water at ambient conditions. The catalysts and the products (symmetrical biaryls) were characterized using their spectral data. The high yields of the biaryls, the bio-mimicking conditions, good substrate feasibility, evading the use of base, easy preparation and handling of catalysts, and the application of aqueous media, all make this protocol very attractive from a sustainability and cost-effective standpoint.
Keywords: nature-inspired conditions; porphyrin N-pincer Pd(II)-complexes; potassium aryltrifluoroborates; self-coupling; symmetrical biaryls; water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Feng L., Wang K.-Y., Joseph E., Zhou H.-C. Catalytic porphyrin framework compounds. Trends Chem. 2020;2:555–568. doi: 10.1016/j.trechm.2020.01.003. - DOI
-
- Venkateswarlu K., Rao K.U. Cu(OAc)2-porphyrins as an efficient catalytic system for base-free, nature mimicking Chan-Lam coupling in water. Appl. Organometal. Chem. 2021;35:e6223. doi: 10.1002/aoc.6223. - DOI
-
- Mauzerall D. Porphyrins, chlorophyll, and photosynthesis. In: Trebst A., Avron M., editors. Photosynthesis I. Encyclopedia of Plant Physiology (New Series) Volume 117 Springer; Berlin/Heidelberg, Germany: 1997.
-
- Bonkovsky H.L., Guo J.-T., Hou W., Li T., Narang T., Thapar M. Porphyrin and heme metabolism and the porphyrias. Compr. Physiol. 2013;3:365–401. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

