A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water
- PMID: 34501084
- PMCID: PMC8433637
- DOI: 10.3390/ma14174994
A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water
Abstract
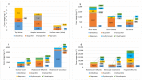
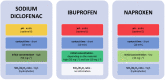
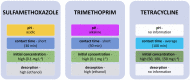
Zeolites and mesoporous silica materials are effective adsorbents that can be useful for the removal of various pharmaceuticals including non-steroidal anti-inflammatory drugs and antibiotics from low-quality water. This paper summarizes the properties and basic characteristics of zeolites and mesoporous silica materials and reviews the recent studies on the efficacy of the adsorption of selected non-steroidal medicinal products and antibiotics by these adsorbents to assess the potential opportunities and challenges of using them in water treatment. It was found that the adsorption capacity of sorbents with high silica content is related to their surface hydrophobicity (hydrophilicity) and structural features, such as micropore volume and pore size, as well as the properties of the studied medicinal products. This review can be of help to scientists to develop an effective strategy for reducing the amount of these two groups of pharmaceuticals in wastewater.
Keywords: drug analysis; mesoporous sorbents; wastewater purification; zeolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

