SHS-Derived Powders by Reactions' Coupling as Primary Products for Subsequent Consolidation
- PMID: 34501207
- PMCID: PMC8434033
- DOI: 10.3390/ma14175117
SHS-Derived Powders by Reactions' Coupling as Primary Products for Subsequent Consolidation
Abstract
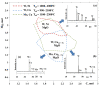
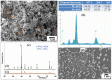
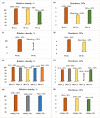
The capability of self-propagating high-temperature synthesis (SHS) to produce powders that are characterized by a high sintering ability, owing to high heating and cooling rates inherent to the exothermic reaction, is of a special interest for the industry. In particular, SHS-derived powders comprise a significant defect concentration in order to effectively enhance the mass transfer processes during the sintering, which allows for the successful consolidation of difficult-to-sinter materials at relatively low sintering temperatures. From this perspective, the design of precursors suitable for sintering, synthesis in a controlled temperature regime and the optimization of geometrical and structural parameters of SHS powders as a potential feedstock for the consolidation is of key importance. Here, we report on the comparative studies concerning the SHS processing of composites for advanced powder metallurgy techniques. The synthesis and sintering peculiarities of the SHS through coupled reactions in the Me'O3(WO3,MoO3)-Me''O(CuO,NiO)-Mg-C, Ti-B-Al12Mg17 systems are comparatively reviewed. The SHS coupling approach was used for the preparation of powders with a tuned degree of fineness (a high specific surface area of particles), a high-homogeneity and a controllable distribution of elements via both the regulation of the thermal regime of combustion in a wide range and the matching of the thermal and kinetic requirements of two interconnected reactions. Microstructural features of the powder feedstock greatly contributed to the subsequent consolidation process.
Keywords: combustion synthesized powder; mechanical properties; microstructure; self-propagating high-temperature synthesis; sintering; thermal coupling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Merzhanov A.G. History and recent developments in SHS. Ceram. Int. 1995;21:371–379. doi: 10.1016/0272-8842(95)96211-7. - DOI
-
- Varma A., Rogachev A.S., Mukasyan A.S., Hwang S. Advances in Chemical Engineering. Volume 24. Academic Press; Cambridge, MA, USA: 1998. Combustion synthesis of advanced materials: Principles and applications; pp. 79–226.
-
- Liu G., Li J., Chen K. Combustion synthesis of refractory and hard materials: A review. Int. J. Ref. Metals Hard Mater. 2013;39:90–102. doi: 10.1016/j.ijrmhm.2012.09.002. - DOI
-
- Mishra S.K., Das S., Pathak L.C. Defect structures in zirconium diboride powder prepared by self-propagating high-temperature synthesis. Mat. Sci. Eng. A. 2004;364:249–255. doi: 10.1016/j.msea.2003.08.021. - DOI
-
- Orrù R., Cao G. Concise Encyclopedia of Self-Propagating High-Temperature Synthesis. Elsevier; Amsterdam, The Netherlands: 2017. Spark Plasma Sintering of SHS Powders; pp. 349–351.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

